Prevalence and clinical characteristics of non-malignant CT detected incidental findings in the SUMMIT lung cancer screening cohort
- PMID: 37321665
- PMCID: PMC10277548
- DOI: 10.1136/bmjresp-2023-001664
Prevalence and clinical characteristics of non-malignant CT detected incidental findings in the SUMMIT lung cancer screening cohort
Abstract
Background: Pulmonary and extrapulmonary incidental findings are frequently identified on CT scans performed for lung cancer screening. Uncertainty regarding their clinical significance and how and when such findings should be reported back to clinicians and participants persists. We examined the prevalence of non-malignant incidental findings within a lung cancer screening cohort and investigated the morbidity and relevant risk factors associated with incidental findings. We quantified the primary and secondary care referrals generated by our protocol.
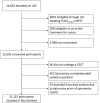
Methods: The SUMMIT study (NCT03934866) is a prospective observational cohort study to examine the performance of delivering a low-dose CT (LDCT) screening service to a high-risk population. Spirometry, blood pressure, height/weight and respiratory history were assessed as part of a Lung Health Check. Individuals at high risk of lung cancer were offered an LDCT and returned for two further annual visits. This analysis is a prospective evaluation of the standardised reporting and management protocol for incidental findings developed for the study on the baseline LDCT.
Results: In 11 115 participants included in this analysis, the most common incidental findings were coronary artery calcification (64.2%) and emphysema (33.4%). From our protocolised management approach, the number of participants requiring review for clinically relevant findings in primary care was 1 in 20, and the number potentially requiring review in secondary care was 1 in 25.
Conclusions: Incidental findings are common in lung cancer screening and can be associated with reported symptoms and comorbidities. A standardised reporting protocol allows systematic assessment and standardises onward management.
Keywords: Imaging/CT MRI etc; Lung Cancer.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ST, AWC, JD, CH, HH and PV are all funded or part-funded through GRAIL as part of the SUMMIT Study. SJ was a Wellcome Trust Senior Fellow in Clinical Science (WT107963AIA). SJ is supported by CRUK programme grant EDDCPGM\100002, the Rosetrees Trust, the Roy Castle Lung Cancer foundation, the Garfield Weston Trust and UCLH Charitable Foundation. SJ’s full disclosures are as a Paid Advisory Board member Astra-Zeneca, Bard1 Bioscience, Achilles Therapeutics, Jansen. Assistance for travel to meetings from Astra Zeneca, Takeda, and grant income from GRAIL, Owlstone and share options from Optellum; BARD1 Lifescience. SQ is supported by a Cancer Research UK (CRUK) Population Research Fellowship (C50664/A24460) and Barts Charity (MRC&U0036). AN is part-funded through the UCLH Biomedical Research Centre. AD’s disclosures are personal fees from Boehringer Ingelheim, Roche, Galacto Biotech, Galapagos and Vicore. AH’s disclosures are consulting fees to Evidera and assistance for travel to meetings from GRAIL. JRH’s disclosures are assistance for travel from Astra Zeneca and Boehringer Ingelheim and payment for lectures and presentations from Astra Zeneca, Boehringer Ingelheim, Nutricia and Takeda. There are no disclosures from KG, VB, CL, A-MH, JT and LF.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical