Change in cardiorespiratory parameters following surgical correction of pectus excavatum: protocol for the historical-prospective HeartSoar cohort
- PMID: 37321811
- PMCID: PMC10277089
- DOI: 10.1136/bmjopen-2022-070891
Change in cardiorespiratory parameters following surgical correction of pectus excavatum: protocol for the historical-prospective HeartSoar cohort
Abstract
Introduction: How cardiorespiratory function changes following the surgical correction of pectus excavatum (PE) often gives mixed results, with meta-analyses demonstrating no benefit in terms of pulmonary function but improvement in cardiac function. Functional responses may depend on type of surgery, follow-up time and/or the patient's presurgical functional status, and debate persists on the purely aesthetic nature of such surgery. The aim of this protocol is to analyse data describing lung function and incremental exercise testing before vs after the surgical correction of PE.
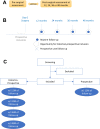
Methods and analysis: A historical-prospective before-after surgical correction of PE cohort will be constituted. Historical inclusions are recruited during follow-up visits at approximately 12, 24, 36 or 48 months following a prior surgery (with presurgical data mined from patient records). Prospective inclusions are recruited during presurgical work-ups and followed for 1 year following surgery. The data collected include spirometry, incremental exercise testing, body mass index, body composition, questionnaires targeting general health status, self-esteem and body image. Any complications due to surgery are also described.The primary outcome is oxygen pulse during incremental exercise testing, and 44 data points are required to demonstrate a moderate postsurgical change (ie, a Cohen's effect of d=0.5). Wilcoxon signed-rank tests or t-tests for paired data will be used for before-after comparisons (with false discovery rate corrections for secondary analyses).
Ethics and dissemination: This study will be conducted according to the principles of the Declaration of Helsinki (as revised in 2013) and was approved by a randomly assigned, independent, ethics committee (Comité de Protection des Personnes Sud-Méditerranée II, reference number: 218 B21) as per French law on 6 July 2018. Informed, written consent for study participation is required of all study candidates prior to enrolment. Results will be published in an international peer-reviewed journal.
Trial registration number: NCT03770390; Clinicaltrials.gov.
Keywords: musculoskeletal disorders; respiratory physiology; thoracic surgery.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AB reports grants, personal fees, non-financial support and other from Astra Zeneca, grants, personal fees and other from GSK, grants, personal fees, non-financial support and other from Boeringher Ingelheim, personal fees, non-financial support and other from Novartis, personal fees and other from Teva, personal fees and other from Regeneron, personal fees, non-financial support and other from Chiesi Farmaceuticals, personal fees, non-financial support and other from Actelion, other from Gilead, personal fees and non-financial support from Roche, outside the submitted work. CMS reports a previous unrelated grant and personal fees from Astra Zeneca. NM reports personal fees from Astra Zeneca and an unrelated grant from GSK. LS has no conflicts of interest to declare.
Figures
Similar articles
-
Cardiorespiratory function is significantly improved following corrective surgery for severe pectus excavatum. Proposed treatment guidelines.J Cardiovasc Surg (Torino). 2000 Feb;41(1):125-30. J Cardiovasc Surg (Torino). 2000. PMID: 10836238
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
The questionable benefit of pectus excavatum repair on cardiopulmonary function: a prospective study.Eur J Cardiothorac Surg. 2021 Dec 27;61(1):75-82. doi: 10.1093/ejcts/ezab296. Eur J Cardiothorac Surg. 2021. PMID: 34263302 Free PMC article. Clinical Trial.
-
Pectus Excavatum: Consensus and Controversies in Clinical Practice.Ann Thorac Surg. 2023 Jul;116(1):191-199. doi: 10.1016/j.athoracsur.2023.02.059. Epub 2023 Mar 29. Ann Thorac Surg. 2023. PMID: 36997016 Review.
-
The physiologic impact of pectus excavatum repair.Semin Pediatr Surg. 2018 Jun;27(3):127-132. doi: 10.1053/j.sempedsurg.2018.05.005. Semin Pediatr Surg. 2018. PMID: 30078483 Review.
Cited by
-
Functional and Aesthetic Outcomes of Patients Underwent Modified Ravitch Technique for Repair of Pectus Excavatum.Med J Islam Repub Iran. 2024 Aug 20;38:95. doi: 10.47176/mjiri.38.95. eCollection 2024. Med J Islam Repub Iran. 2024. PMID: 39678765 Free PMC article.
References
-
- Steinmann C, Krille S, Mueller A, et al. . Pectus Excavatum and Pectus Carinatum patients suffer from lower quality of life and impaired body image: a control group comparison of psychological characteristics prior to surgical correction. Eur J Cardiothorac Surg 2011;40:1138–45. 10.1016/j.ejcts.2011.02.019 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
