Histopathology reporting of temporal artery biopsy specimens for giant cell arteritis: results of a modified Delphi study
- PMID: 37321853
- PMCID: PMC11228225
- DOI: 10.1136/jcp-2023-208810
Histopathology reporting of temporal artery biopsy specimens for giant cell arteritis: results of a modified Delphi study
Abstract
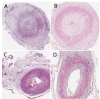
The temporal artery biopsy (TAB) is regarded as the gold-standard test in the diagnosis of giant cell arteritis (GCA). There is a lack of agreement among experienced pathologists regarding the diagnostic features and classification of inflammation observed in TAB sections in the diagnosis of GCA.
Aims: The aim of this research study was to establish consensus on the key parameters which should be included in a standardised reporting proforma for TAB specimens. We specifically investigated factors pertaining to clinical information, specimen handling and microscopic pathological features.
Methods: A modified Delphi process, comprising three survey rounds and three virtual consensus group meetings, was undertaken by 13 UK-based pathology or ophthalmology consultants, with a 100% response rate across the three rounds. Initial statements were formulated after a literature review and participants were asked to rate their agreement using a nine-point Likert scale. Consensus was defined a priori as an agreement of ≥70% and individual feedback was provided after each round, together with data on the distribution of group responses.
Results: Overall, 67 statements reached consensus and 17 statements did not. The participants agreed on the core microscopic features to be included in a pathology report and felt that a proforma would facilitate consistent reporting practices.
Conclusions: Our work revealed uncertainty surrounding the correlation between clinical parameters (eg, laboratory markers of inflammation and steroid therapy duration) and microscopic findings, and we propose areas for future research.
Keywords: DIAGNOSIS; INFLAMMATION; NEUROPATHOLOGY; Pathology, Surgical; VASCULITIS.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: SLM reports consultancy on behalf of her institution for Roche/Chugai, Sanofi, AbbVie and AstraZeneca; speaker fees received by her institution from Pfizer, Chugai and Vifor; and support from Roche to attend EULAR2019 and Pfizer to attend ACR2021.
Figures
References
-
- National Institute for Health and Care Excellence . Technology appraisal guidance Ta518. In: Tocilizumab for treating giant cell arteritis. London: NICE, 2018. Available: https:// www.nice.org.uk/guidance/ta518
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical