Albumosomes formed by cytoplasmic pre-folding albumin maintain mitochondrial homeostasis and inhibit nonalcoholic fatty liver disease
- PMID: 37321990
- PMCID: PMC10272166
- DOI: 10.1038/s41392-023-01437-0
Albumosomes formed by cytoplasmic pre-folding albumin maintain mitochondrial homeostasis and inhibit nonalcoholic fatty liver disease
Abstract
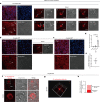
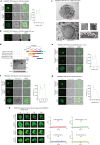
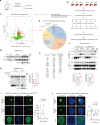
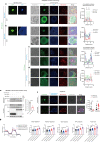
Hepatic mitochondrial dysfunction contributes to the progression of nonalcoholic fatty liver disease (NAFLD). However, the factors that maintain mitochondrial homeostasis, especially in hepatocytes, are largely unknown. Hepatocytes synthesize various high-level plasma proteins, among which albumin is most abundant. In this study, we found that pre-folding albumin in the cytoplasm is completely different from folded albumin in the serum. Mechanistically, endogenous pre-folding albumin undergoes phase transition in the cytoplasm to form a shell-like spherical structure, which we call the "albumosome". Albumosomes interact with and trap pre-folding carnitine palmitoyltransferase 2 (CPT2) in the cytoplasm. Albumosomes control the excessive sorting of CPT2 to the mitochondria under high-fat-diet-induced stress conditions; in this way, albumosomes maintain mitochondrial homeostasis from exhaustion. Physiologically, albumosomes accumulate in hepatocytes during murine aging and protect the livers of aged mice from mitochondrial damage and fat deposition. Morphologically, mature albumosomes have a mean diameter of 4μm and are surrounded by heat shock protein Hsp90 and Hsp70 family proteins, forming a larger shell. The Hsp90 inhibitor 17-AAG promotes hepatic albumosomal accumulation in vitro and in vivo, through which suppressing the progression of NAFLD in mice.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

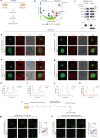
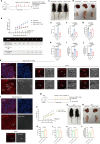
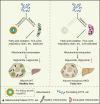
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

