Mid-infrared chemical imaging of intracellular tau fibrils using fluorescence-guided computational photothermal microscopy
- PMID: 37322011
- PMCID: PMC10272128
- DOI: 10.1038/s41377-023-01191-6
Mid-infrared chemical imaging of intracellular tau fibrils using fluorescence-guided computational photothermal microscopy
Abstract
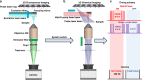
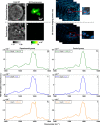
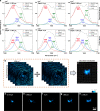
Amyloid proteins are associated with a broad spectrum of neurodegenerative diseases. However, it remains a grand challenge to extract molecular structure information from intracellular amyloid proteins in their native cellular environment. To address this challenge, we developed a computational chemical microscope integrating 3D mid-infrared photothermal imaging with fluorescence imaging, termed Fluorescence-guided Bond-Selective Intensity Diffraction Tomography (FBS-IDT). Based on a low-cost and simple optical design, FBS-IDT enables chemical-specific volumetric imaging and 3D site-specific mid-IR fingerprint spectroscopic analysis of tau fibrils, an important type of amyloid protein aggregates, in their intracellular environment. Label-free volumetric chemical imaging of human cells with/without seeded tau fibrils is demonstrated to show the potential correlation between lipid accumulation and tau aggregate formation. Depth-resolved mid-infrared fingerprint spectroscopy is performed to reveal the protein secondary structure of the intracellular tau fibrils. 3D visualization of the β-sheet for tau fibril structure is achieved.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
Mid-infrared Chemical Imaging of Intracellular Tau Fibrils using Fluorescence-guided Computational Photothermal Microscopy.ArXiv [Preprint]. 2023 Feb 23:arXiv:2302.11769v1. ArXiv. 2023. Update in: Light Sci Appl. 2023 Jun 15;12(1):147. doi: 10.1038/s41377-023-01191-6. PMID: 36866226 Free PMC article. Updated. Preprint.
Similar articles
-
Mid-infrared Chemical Imaging of Intracellular Tau Fibrils using Fluorescence-guided Computational Photothermal Microscopy.ArXiv [Preprint]. 2023 Feb 23:arXiv:2302.11769v1. ArXiv. 2023. Update in: Light Sci Appl. 2023 Jun 15;12(1):147. doi: 10.1038/s41377-023-01191-6. PMID: 36866226 Free PMC article. Updated. Preprint.
-
Bond-selective intensity diffraction tomography.Nat Commun. 2022 Dec 15;13(1):7767. doi: 10.1038/s41467-022-35329-8. Nat Commun. 2022. PMID: 36522316 Free PMC article.
-
Fluorescence-Detected Mid-Infrared Photothermal Microscopy.J Am Chem Soc. 2021 Aug 4;143(30):11490-11499. doi: 10.1021/jacs.1c03642. Epub 2021 Jul 15. J Am Chem Soc. 2021. PMID: 34264654 Free PMC article.
-
Exploring the structure and formation mechanism of amyloid fibrils by Raman spectroscopy: a review.Analyst. 2015 Aug 7;140(15):4967-80. doi: 10.1039/c5an00342c. Analyst. 2015. PMID: 26042229 Review.
-
Techniques to study amyloid fibril formation in vitro.Methods. 2004 Sep;34(1):151-60. doi: 10.1016/j.ymeth.2004.03.012. Methods. 2004. PMID: 15283924 Review.
Cited by
-
Structural characterization of amyloid aggregates with spatially resolved infrared spectroscopy.Methods Enzymol. 2024;697:113-150. doi: 10.1016/bs.mie.2024.02.013. Epub 2024 Apr 5. Methods Enzymol. 2024. PMID: 38816120 Free PMC article.
-
Strain-Distinct α-Synuclein and Tau Cross-Seeding Uncovered by Correlative Approach with Optical Photothermal Infrared Sub-Micron Imaging.J Am Chem Soc. 2025 Aug 6;147(31):27323-27340. doi: 10.1021/jacs.5c02811. Epub 2025 Jul 28. J Am Chem Soc. 2025. PMID: 40726080 Free PMC article.
-
Aggregation, Transmission, and Toxicity of the Microtubule-Associated Protein Tau: A Complex Comprehension.Int J Mol Sci. 2023 Oct 9;24(19):15023. doi: 10.3390/ijms241915023. Int J Mol Sci. 2023. PMID: 37834471 Free PMC article. Review.
-
Structural diversity of Alzheimer-related protein aggregations revealed using photothermal ratio-metric micro-spectroscopy.Biomed Opt Express. 2024 Nov 11;15(12):6768-6782. doi: 10.1364/BOE.537461. eCollection 2024 Dec 1. Biomed Opt Express. 2024. PMID: 39679398 Free PMC article.
-
Lipid mediated formation of antiparallel aggregates in cerebral amyloid angiopathy.Acta Neuropathol. 2025 Jul 13;150(1):3. doi: 10.1007/s00401-025-02911-5. Acta Neuropathol. 2025. PMID: 40652435 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

