AAV-mediated expression of a new conformational anti-aggregated α-synuclein antibody prolongs survival in a genetic model of α-synucleinopathies
- PMID: 37322068
- PMCID: PMC10272115
- DOI: 10.1038/s41531-023-00542-9
AAV-mediated expression of a new conformational anti-aggregated α-synuclein antibody prolongs survival in a genetic model of α-synucleinopathies
Abstract
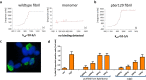
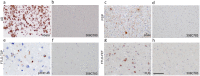
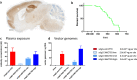
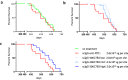
Prion-like transmission of pathology in α-synucleinopathies like Parkinson's disease or multiple system atrophy is increasingly recognized as one potential mechanism to address disease progression. Active and passive immunotherapies targeting insoluble, aggregated α-synuclein are already being actively explored in the clinic with mixed outcomes so far. Here, we report the identification of 306C7B3, a highly selective, aggregate-specific α-synuclein antibody with picomolar affinity devoid of binding to the monomeric, physiologic protein. 306C7B3 binding is Ser129-phosphorylation independent and shows high affinity to several different aggregated α-synuclein polymorphs, increasing the likelihood that it can also bind to the pathological seeds assumed to drive disease progression in patients. In support of this, highly selective binding to pathological aggregates in postmortem brains of MSA patients was demonstrated, with no staining in samples from other human neurodegenerative diseases. To achieve CNS exposure of 306C7B3, an adeno-associated virus (AAV) based approach driving expression of the secreted antibody within the brain of (Thy-1)-[A30P]-hα-synuclein mice was used. Widespread central transduction after intrastriatal inoculation was ensured by using the AAV2HBKO serotype, with transduction being spread to areas far away from the inoculation site. Treatment of (Thy-1)-[A30P]-hα-synuclein mice at the age of 12 months demonstrated significantly increased survival, with 306C7B3 concentration reaching 3.9 nM in the cerebrospinal fluid. These results suggest that AAV-mediated expression of 306C7B3, targeting extracellular, presumably disease-propagating aggregates of α-synuclein, has great potential as a disease-modifying therapy for α-synucleinopathies as it ensures CNS exposure of the antibody, thereby mitigating the selective permeability of the blood-brain barrier.
© 2023. The Author(s).
Conflict of interest statement
None of the authors declare a competing financial or non-financial interest. All authors except M.N., R.M. and J.S. were employed by Boehringer Ingelheim, a privately owned pharmaceutical company, at the time this work was executed. Nobody holds stock or stock options of Boehringer Ingelheim. No patent applications have been filed related to this work.
Figures






Similar articles
-
ABBV-0805, a novel antibody selective for soluble aggregated α-synuclein, prolongs lifespan and prevents buildup of α-synuclein pathology in mouse models of Parkinson's disease.Neurobiol Dis. 2021 Dec;161:105543. doi: 10.1016/j.nbd.2021.105543. Epub 2021 Nov 1. Neurobiol Dis. 2021. PMID: 34737044
-
Comparative analyses of the in vivo induction and transmission of α-synuclein pathology in transgenic mice by MSA brain lysate and recombinant α-synuclein fibrils.Acta Neuropathol Commun. 2019 May 20;7(1):80. doi: 10.1186/s40478-019-0733-3. Acta Neuropathol Commun. 2019. PMID: 31109378 Free PMC article.
-
Prion-like propagation of human brain-derived alpha-synuclein in transgenic mice expressing human wild-type alpha-synuclein.Acta Neuropathol Commun. 2015 Nov 26;3:75. doi: 10.1186/s40478-015-0254-7. Acta Neuropathol Commun. 2015. PMID: 26612754 Free PMC article.
-
Pathogenic Impact of α-Synuclein Phosphorylation and Its Kinases in α-Synucleinopathies.Int J Mol Sci. 2022 Jun 1;23(11):6216. doi: 10.3390/ijms23116216. Int J Mol Sci. 2022. PMID: 35682892 Free PMC article. Review.
-
α-Synuclein pathology in Parkinson's disease and related α-synucleinopathies.Neurosci Lett. 2019 Sep 14;709:134316. doi: 10.1016/j.neulet.2019.134316. Epub 2019 Jun 3. Neurosci Lett. 2019. PMID: 31170426 Free PMC article. Review.
Cited by
-
Gene therapy rescues brain edema and motor function in a mouse model of megalencephalic leukoencephalopathy with subcortical cysts.Mol Ther. 2025 Apr 2;33(4):1434-1448. doi: 10.1016/j.ymthe.2025.02.046. Epub 2025 Mar 5. Mol Ther. 2025. PMID: 40051162 Free PMC article.
-
Autonomic dysfunction in multiple system atrophy: from pathophysiology to clinical manifestations.Ann Med. 2025 Dec;57(1):2488111. doi: 10.1080/07853890.2025.2488111. Epub 2025 Apr 8. Ann Med. 2025. PMID: 40719373 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

