Nanosensor-based monitoring of autophagy-associated lysosomal acidification in vivo
- PMID: 37322156
- PMCID: PMC10721723
- DOI: 10.1038/s41589-023-01364-9
Nanosensor-based monitoring of autophagy-associated lysosomal acidification in vivo
Abstract
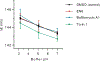
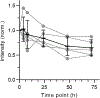
Autophagy is a cellular process with important functions that drive neurodegenerative diseases and cancers. Lysosomal hyperacidification is a hallmark of autophagy. Lysosomal pH is currently measured by fluorescent probes in cell culture, but existing methods do not allow for quantitative, transient or in vivo measurements. In the present study, we developed near-infrared optical nanosensors using organic color centers (covalent sp3 defects on carbon nanotubes) to measure autophagy-mediated endolysosomal hyperacidification in live cells and in vivo. The nanosensors localize to the lysosomes, where the emission band shifts in response to local pH, enabling spatial, dynamic and quantitative mapping of subtle changes in lysosomal pH. Using the sensor, we observed cellular and intratumoral hyperacidification on administration of mTORC1 and V-ATPase modulators, revealing that lysosomal acidification mirrors the dynamics of S6K dephosphorylation and LC3B lipidation while diverging from p62 degradation. This sensor enables the transient and in vivo monitoring of the autophagy-lysosomal pathway.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
D.A.H. is a co-founder and officer with equity interest in Lime Therapeutics, Inc., and co-founder with equity interest in Selectin Therapeutics Inc. and Resident Diagnostics, Inc., and a member of the scientific advisory board of Concarlo Therapeutics, Inc., Nanorobotics Inc., and Mediphage Bioceuticals, Inc. T.T. has research support from ONO Pharma USA., Inc. (unrelated to this work) and is a member of the scientific advisory board of Lime Therapeutics, Inc. with equity interest. The remaining authors declare no competing interests.
Figures

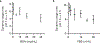
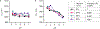









References
-
- Lawrence RE & Zoncu R The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 21, 133–142 (2019). - PubMed
Methods-only references
-
- Subbaiyan NK et al. Role of surfactants and salt in aqueous two-phase separation of carbon nanotubes toward simple chirality isolation. ACS Nano 8, 1619–1628 (2014). - PubMed
-
- Quintero B, Cabeza MC, Martínez MI, Gutiérrez P & Martínez PJ Dediazoniation of p-hydroxy and p-nitrobenzenediazonium ions in an aqueous medium: Interference by the chelating agent diethylenetriaminepentaacetic acid. Can. J. Chem. 81, 832–839 (2003).
-
- Streit JK, Fagan JA & Zheng M A low energy route to DNA-wrapped carbon nanotubes via replacement of bile salt surfactants. Anal. Chem. 89, 10496–10503 (2017). - PubMed
-
- Zheng M & Diner BA Solution redox chemistry of carbon nanotubes. J Am Chem Soc 126, 15490–15494 (2004). - PubMed
-
- Bolte S & CordeliÈRes FP A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

