Effect of Intravenous Golimumab on Fatigue and the Relationship with Clinical Response in Adults with Active Ankylosing Spondylitis in the Phase 3 GO-ALIVE Study
- PMID: 37322274
- PMCID: PMC10326229
- DOI: 10.1007/s40744-023-00556-y
Effect of Intravenous Golimumab on Fatigue and the Relationship with Clinical Response in Adults with Active Ankylosing Spondylitis in the Phase 3 GO-ALIVE Study
Abstract
Introduction: We studied the effect of intravenous (IV)-golimumab on fatigue and the association of fatigue improvement with clinical response post hoc in adults with active ankylosing spondylitis (AS) in the GO-ALIVE trial.
Methods: Patients were randomized to IV-golimumab 2 mg/kg (N = 105) at week (W) 0, W4, then every 8 W (Q8W) or placebo (N = 103) at W0, W4, W12, crossover to IV-golimumab 2 mg/kg at W16, W20, then Q8W through W52. Fatigue measures included Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) Question #1 (fatigue; 0 [none], 10 [worst]; decrease indicates improvement) and 36-Item Short Form Health Survey (SF-36) vitality subscale (0 [worst], 100 [best]; increase indicates improvement). Minimum clinically important difference is ≥ 1 for BASDAI-fatigue and ≥ 5 for SF-36 vitality. GO-ALIVE primary endpoint was Assessment of SpondyloArthritis international Society ≥ 20% improvement criteria (ASAS20). Other clinical outcomes assessed included other ASAS responses, Ankylosing Spondylitis Disease Activity Score, and Bath Ankylosing Spondylitis Functional Index score. The distribution-based minimally important differences (MIDs) were determined for BASDAI-fatigue and SF-36 vitality. The relationship between improvement in fatigue and clinical outcomes was assessed via multivariable logistic regression.
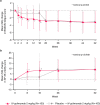
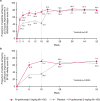
Results: Mean changes in BASDAI-fatigue/SF-36 vitality scores were greater with IV-golimumab versus placebo at W16 (- 2.74/8.46 versus - 0.73/2.08, both nominal p ≤ 0.003); by W52 (after crossover), differences between groups narrowed (- 3.18/9.39 versus - 3.07/9.17). BASDAI-fatigue/SF-36 vitality MIDs were achieved by greater proportions of IV-golimumab-treated versus placebo-treated patients at W16 (75.2%/71.4% versus 42.7%/35.0%). A one-point/five-point improvement in BASDAI-fatigue/SF-36 vitality scores at W16 increased likelihood of achieving ASAS20 (odds ratios [95% confidence intervals]: 3.15 [2.21, 4.50] and 2.10 [1.62, 2.71], respectively) and ASAS40 (3.04 [2.15, 4.28] and 2.24 [1.68, 3.00], respectively) responses at W16; concurrent improvements and clinical response at W52 were consistent. A one-point/five-point improvement in BASDAI-fatigue/SF-36 vitality scores at W16 predicted increased likelihood of achieving ASAS20 (1.62 [1.35, 1.95] and 1.52 [1.25, 1.86], respectively) and ASAS40 (1.62 [1.37, 1.92] and 1.44 [1.20, 1.73], respectively) responses at W52.
Conclusions: IV-golimumab provided important and sustained fatigue improvement in patients with AS that positively associated with achieving clinical response.
Trial registration: ClinicalTrials.gov identifier, NCT02186873.
Keywords: Ankylosing spondylitis; Clinical response; Fatigue; Intravenous golimumab; Vitality.
Plain language summary
Ankylosing spondylitis (AS) is a type of arthritis that mostly affects the spine. Patients with AS also often have severe fatigue. Intravenous (IV)-golimumab, which blocks the inflammatory action of tumor necrosis factor, is approved to treat AS. We used information from a clinical trial (GO-ALIVE) to determine whether IV-golimumab reduced fatigue in patients with AS, and if fatigue improvement was associated with improvement in other AS symptoms, including spinal pain, ability to function, and inflammation. In the 1-year GO-ALIVE study, patients were assigned to receive either IV-golimumab or placebo. Patients assigned to placebo were switched to IV-golimumab starting at week 16. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) fatigue question and the 36-Item Short Form Health Survey (SF-36) vitality subscale were used to assess fatigue. Improvement in AS symptoms was measured using the Assessment of SpondyloArthritis international Society ≥ 20% and ≥ 40% improvement criteria (ASAS20 and ASAS40). After 16 weeks of treatment, patients treated with IV-golimumab, on average, had statistically significantly greater improvement in both measures of fatigue than patients treated with placebo. At 1 year, after the placebo group had received IV-golimumab starting at week 16, improvement in fatigue was similar between groups. Improvement in fatigue at week 16 increased the likelihood that ASAS20 and ASAS40 would be achieved at week 16. Similar results were observed at 1 year. Additionally, improvement in fatigue at week 16 predicted the likelihood of achieving ASAS20 and ASAS40 at 1 year. Together, these results demonstrate that IV-golimumab provided important, long-term improvement in fatigue in patients with AS that was positively associated with improvement in AS symptoms.
© 2023. The Author(s).
Conflict of interest statement
Atul Deodhar has received consulting fees for participation in advisory boards from AbbVie, Amgen, Aurinia, Bristol Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, MoonLake, Novartis, Pfizer, and UCB; grant/research support from AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, MoonLake, Novartis, Pfizer, and UCB; speaker/advisory board fees from AbbVie, Aurinia, Eli Lilly, Janssen, MoonLake, Novartis, Pfizer, and UCB; and medical writing support funded by AbbVie, Amgen, Eli Lilly, Novartis, Pfizer, and UCB. Natalie J. Shiff is an employee of Janssen Scientific Affairs, LLC; received salary support from the Childhood Arthritis and Rheumatology Research Alliance within the past 3 years; and owns or has owned stock in AbbVie, Gilead, Iovance, Novo-Nordisc, and Pfizer within the past 3 years. Cinty Gong is an employee of Janssen Scientific Affairs, LLC. Eric K.H. Chan is an employee of Janssen Global Services, LLC, a subsidiary of Johnson & Johnson, and owns stock in Johnson & Johnson. Elizabeth C. Hsia, Kim Hung Lo, Aliana Akawung, Lilliane Kim, and Stephen Xu are employees of Janssen Research & Development, LLC, a subsidiary of Johnson & Johnson, and may own stock in Johnson & Johnson. John D. Reveille has received consulting fees for participation in advisory boards from Eli Lilly and UCB, and research grant funding from Eli Lilly and Janssen.
Figures

Similar articles
-
Safety and Efficacy of Intravenous Golimumab in Adults with Ankylosing Spondylitis: Results through 1 Year of the GO-ALIVE Study.J Rheumatol. 2019 Oct;46(10):1277-1283. doi: 10.3899/jrheum.180718. Epub 2019 Mar 1. J Rheumatol. 2019. PMID: 30824635 Clinical Trial.
-
Propensity score matching/reweighting analysis comparing intravenous golimumab to infliximab for ankylosing spondylitis using data from the GO-ALIVE and ASSERT trials.Clin Rheumatol. 2020 Oct;39(10):2907-2917. doi: 10.1007/s10067-020-05051-1. Epub 2020 May 4. Clin Rheumatol. 2020. PMID: 32367407 Free PMC article.
-
Translating Improvements with Ixekizumab in Clinical Trial Outcomes into Clinical Practice: ASAS40, Pain, Fatigue, and Sleep in Ankylosing Spondylitis.Rheumatol Ther. 2019 Sep;6(3):435-450. doi: 10.1007/s40744-019-0165-3. Epub 2019 Jun 28. Rheumatol Ther. 2019. PMID: 31254223 Free PMC article.
-
Efficacy and Safety of Wenbu Zhibi Granule in Patients with Ankylosing Spondylitis: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial.Evid Based Complement Alternat Med. 2021 Nov 25;2021:8683600. doi: 10.1155/2021/8683600. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34868333 Free PMC article. Review.
-
Patients with ankylosing spondylitis treatment by golimumab: a systematic review and meta-analysis.Eur Spine J. 2020 Aug;29(8):1813-1822. doi: 10.1007/s00586-020-06466-9. Epub 2020 May 23. Eur Spine J. 2020. Retraction in: Eur Spine J. 2022 Apr;31(4):1067. doi: 10.1007/s00586-022-07129-7. PMID: 32447529 Retracted.
Cited by
-
Patient self-reported experience and satisfaction with golimumab and etanercept treatments for rheumatic diseases: A cohort study.Medicine (Baltimore). 2024 Feb 23;103(8):e36982. doi: 10.1097/MD.0000000000036982. Medicine (Baltimore). 2024. PMID: 38394542 Free PMC article.
References
-
- Connolly D, Fitzpatrick C, O’Shea F. Disease activity, occupational participation, and quality of life for individuals with and without severe fatigue in ankylosing spondylitis. Occup Ther Int. 2019. https://www.hindawi.com/journals/oti/2019/3027280/. - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

