Latozinemab, a novel progranulin-elevating therapy for frontotemporal dementia
- PMID: 37322482
- PMCID: PMC10268535
- DOI: 10.1186/s12967-023-04251-y
Latozinemab, a novel progranulin-elevating therapy for frontotemporal dementia
Abstract
Background: Heterozygous loss-of-function mutations in the progranulin (PGRN) gene (GRN) cause a reduction in PGRN and lead to the development of frontotemporal dementia (FTD-GRN). PGRN is a secreted lysosomal chaperone, immune regulator, and neuronal survival factor that is shuttled to the lysosome through multiple receptors, including sortilin. Here, we report the characterization of latozinemab, a human monoclonal antibody that decreases the levels of sortilin, which is expressed on myeloid and neuronal cells and shuttles PGRN to the lysosome for degradation, and blocks its interaction with PGRN.
Methods: In vitro characterization studies were first performed to assess the mechanism of action of latozinemab. After the in vitro studies, a series of in vivo studies were performed to assess the efficacy of a mouse-cross reactive anti-sortilin antibody and the pharmacokinetics, pharmacodynamics, and safety of latozinemab in nonhuman primates and humans.
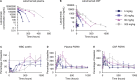
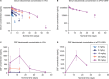
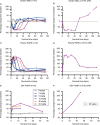
Results: In a mouse model of FTD-GRN, the rodent cross-reactive anti-sortilin antibody, S15JG, decreased total sortilin levels in white blood cell (WBC) lysates, restored PGRN to normal levels in plasma, and rescued a behavioral deficit. In cynomolgus monkeys, latozinemab decreased sortilin levels in WBCs and concomitantly increased plasma and cerebrospinal fluid (CSF) PGRN by 2- to threefold. Finally, in a first-in-human phase 1 clinical trial, a single infusion of latozinemab caused a reduction in WBC sortilin, tripled plasma PGRN and doubled CSF PGRN in healthy volunteers, and restored PGRN to physiological levels in asymptomatic GRN mutation carriers.
Conclusions: These findings support the development of latozinemab for the treatment of FTD-GRN and other neurodegenerative diseases where elevation of PGRN may be beneficial. Trial registration ClinicalTrials.gov, NCT03636204. Registered on 17 August 2018, https://clinicaltrials.gov/ct2/show/NCT03636204 .
Keywords: Frontotemporal dementia; Latozinemab; Neuroprotection; Pharmacodynamics; Pharmacokinetics; Phase 1 clinical trial; Progranulin; Safety; Sortilin.
© 2023. The Author(s).
Conflict of interest statement
A.M., H.L., and A.R. are employees of Alector LLC and may have an equity interest in Alector, Inc. M.K., T.S., R.P., M.W., and F.Y. were Alector employees at the time of manuscript conception and may have an equity interest in Alector, Inc. E.D.R. has served as a consultant to AGTC and as a DSMB member for Lilly.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

