Optogenetic stimulation of basal forebrain cholinergic neurons prevents neuroinflammation and neuropsychiatric manifestations in pristane induced lupus mice
- PMID: 37322485
- PMCID: PMC10268425
- DOI: 10.1186/s12993-023-00213-y
Optogenetic stimulation of basal forebrain cholinergic neurons prevents neuroinflammation and neuropsychiatric manifestations in pristane induced lupus mice
Abstract
Background: Neuroinflammation has been identified as one of the primary pathogenic factors of neuropsychiatric systemic lupus erythematosus (NPSLE). However, there are no dedicated treatments available in clinics to alleviate neuroinflammation in NPSLE. It has been proposed that stimulating basal forebrain (BF) cholinergic neurons may provide potent anti-inflammatory effects in several inflammatory diseases, but its potential role in NPSLE remains unexplored. This study aims to investigate whether and how stimulating BF cholinergic neurons has a protective effect on NPSLE.
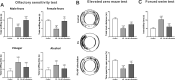
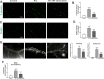
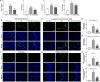
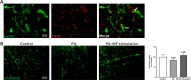
Results: Optogenetic stimulation of BF cholinergic neurons significantly ameliorated olfactory dysfunction and anxiety- and depression-like phenotype in pristane induced lupus (PIL) mice. The increased expression of adhesion molecules (P-selectin and vascular cell adhesion molecule-1 (VCAM-1)), leukocyte recruitment, blood-brain barrier (BBB) leakage were significantly decreased. Notably, the brain histopathological changes, including the elevated levels of pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β), IgG deposition in the choroid plexus and lateral ventricle wall and lipofuscin accumulation in the cortical and hippocampal neurons, were also significantly attenuated. Furthermore, we confirmed the colocalization between the BF cholinergic projections and the cerebral vessels, and the expression of α7-nicotinic acetylcholine receptor (α7nAChR) on the cerebral vessels.
Conclusion: Our data indicate that stimulation of BF cholinergic neurons could play a neuroprotective role in the brain through its cholinergic anti-inflammatory effects on cerebral vessels. Therefore, this may be a promising preventive target for NPSLE.
Keywords: Basal forebrain; Behavioral deficits; Cholinergic anti-inflammatory effect; Neuroinflammation; Neuropsychiatric lupus; Optogenetics; α7nAChR.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Not applicable.
Figures

Similar articles
-
Pristane induced lupus mice as a model for neuropsychiatric lupus (NPSLE).Behav Brain Funct. 2023 Feb 10;19(1):3. doi: 10.1186/s12993-023-00205-y. Behav Brain Funct. 2023. PMID: 36765366 Free PMC article.
-
Cholinergic Neurons in the Basal Forebrain Promote Wakefulness by Actions on Neighboring Non-Cholinergic Neurons: An Opto-Dialysis Study.J Neurosci. 2016 Feb 10;36(6):2057-67. doi: 10.1523/JNEUROSCI.3318-15.2016. J Neurosci. 2016. PMID: 26865627 Free PMC article.
-
Disturbance of neuron-microglia crosstalk mediated by GRP78 in Neuropsychiatric systemic lupus erythematosus mice.J Neuroinflammation. 2023 Jun 26;20(1):150. doi: 10.1186/s12974-023-02832-8. J Neuroinflammation. 2023. PMID: 37365565 Free PMC article.
-
Optogenetic Dissection of the Basal Forebrain Neuromodulatory Control of Cortical Activation, Plasticity, and Cognition.J Neurosci. 2015 Oct 14;35(41):13896-903. doi: 10.1523/JNEUROSCI.2590-15.2015. J Neurosci. 2015. PMID: 26468190 Free PMC article. Review.
-
Lupus animal models and neuropsychiatric implications.Clin Rheumatol. 2021 Jul;40(7):2535-2545. doi: 10.1007/s10067-020-05493-7. Epub 2020 Nov 6. Clin Rheumatol. 2021. PMID: 33155159 Review.
Cited by
-
WIF-1 contributes to lupus-induced neuropsychological deficits via the CRYAB/STAT4-SHH axis.Arthritis Res Ther. 2024 Oct 23;26(1):183. doi: 10.1186/s13075-024-03420-8. Arthritis Res Ther. 2024. PMID: 39444000 Free PMC article.
-
The role of vitamin D: a promising pathway to combat neuropsychiatric lupus disorders.Clin Exp Immunol. 2025 Jan 21;219(1):uxae099. doi: 10.1093/cei/uxae099. Clin Exp Immunol. 2025. PMID: 39495653
-
[Application of optogenetic technology in the research on olfactory bulb neural projection from advanced brain regions to regulate olfactory signal processing].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2024 Dec 25;41(6):1265-1270. doi: 10.7507/1001-5515.202404009. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2024. PMID: 40000218 Free PMC article. Review. Chinese.
References
-
- Morales JY, Young-Stubbs CM, Shimoura CG, Kem WR, Uteshev VV, Mathis KW. Systemic administration of α7-nicotinic acetylcholine receptor ligands does not improve renal injury or behavior in mice with advanced systemic lupus erythematosus. Front Med. 2021;8:642960. doi: 10.3389/fmed.2021.642960. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2021M703606/the fellowship of China Postdoctoral Science Foundation
- 19-109-4-15/the Project for Construction of Key Platform, Shenyang, China
- XLYC2002062/the Xingliao Talent Plan of Liaoning, China
- XLYC2002094/the Xingliao Talent Plan of Liaoning, China
- 2021YFC2501303/the Chinese National Key Technology R&D Program
LinkOut - more resources
Full Text Sources
Miscellaneous

