Effect of Dupilumab on Type 2 Biomarkers in Chronic Rhinosinusitis With Nasal Polyps: SINUS-52 Study Results
- PMID: 37322842
- PMCID: PMC10571440
- DOI: 10.1177/00034894231176334
Effect of Dupilumab on Type 2 Biomarkers in Chronic Rhinosinusitis With Nasal Polyps: SINUS-52 Study Results
Abstract
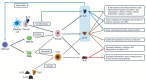
Objectives: Chronic rhinosinusitis with nasal polyps (CRSwNP), asthma, and non-steroidal anti-inflammatory drug-exacerbated respiratory disease (NSAID-ERD) are frequent coexisting conditions and share type 2 inflammatory pathophysiology, with interleukin (IL)-4 and IL-13 as key cytokines. Dupilumab is a monoclonal antibody that blocks the shared receptor for IL-4 and IL-13. The objective of this analysis was to evaluate dupilumab's effect on type 2 inflammation biomarkers in patients with CRSwNP with/without coexisting asthma or NSAID-ERD from the SINUS-52 (NCT02898454) study.
Methods: Patients received treatment with dupilumab or placebo for 52 weeks. Blood and urinary biomarkers were evaluated through 52 weeks, and nasal secretions and mucosa brushings through 24 weeks.
Results: Of 447 patients, 60% had coexisting asthma and 27% had coexisting NSAID-ERD. At baseline, blood eotaxin-3, eosinophils, and periostin, nasal secretion eotaxin-3, and urinary leukotriene E4 were significantly higher in patients with coexisting NSAID-ERD than without. Dupilumab reduced eotaxin-3, thymus and activation-regulated chemokine, periostin, and total immunoglobulin E in blood, eotaxin-3, periostin, IL-5, and eosinophil cationic protein in nasal secretions, and leukotriene E4 in urine. Reductions were generally similar or greater in the subgroups with asthma and NSAID-ERD than without. Dupilumab also reduced MUC5AC and mast cell counts in nasal mucosa brushings.
Conclusion: Dupilumab reduced local and systemic type 2 inflammatory biomarkers in patients with CRSwNP, including mast cells in nasal mucosa and cysteinyl leukotrienes in urine. These findings provide insight into the processes driving CRSwNP and the mechanisms of dupilumab's therapeutic effects.
Clinical trial registry name: SINUS-52 https://www.clinicaltrials.gov/ct2/show/NCT02898454.
Clinicaltrials.gov identifier: NCT02898454.
Keywords: CRSwNP; NSAID-ERD; asthma; biomarkers; comorbidities; dupilumab; type 2 inflammation.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C Bachert reports advisory board fees from ALK, ASIT Biotech, AstraZeneca, GlaxoSmithKline, Intrexon Actobiotics, Novartis, Sanofi, and Stallergenes Greer. TM Laidlaw reports advisory board fees from GlaxoSmithKline, Novartis, Regeneron Pharmaceuticals Inc., and Sanofi. SH Cho reports research grants from National Institutes of Health, Regeneron Pharmaceuticals Inc., and Sanofi. J Mullol has received speaker fees from GlaxoSmithKline, Menarini, Merck Sharp & Dohme, Mylan-Meda Pharmaceuticals (Viatris), Novartis, Regeneron Pharmaceuticals Inc., Sanofi, UCB Pharma, and Uriach; clinical trial funding from Genentech-Roche, Regeneron Pharmaceuticals Inc., and Sanofi; research grants from Mylan-Meda Pharmaceuticals (Viatris) and Uriach; and advisory board fees from Genentech-Roche, Mylan-Meda Pharmaceuticals (Viatris), Novartis, Regeneron Pharmaceuticals Inc., Sanofi, and Uriach. S Naimi, A Jagerschmidt, E Laws, A Praestgaard, and LP Mannent are employees and may hold stock and/or stock options in Sanofi. BN Swanson and M Classe are former employees and may hold stock and/or stock options in Sanofi. S Harel, M Ruddy, and N Amin are former employees and may hold stock and/or stock options in Regeneron Pharmaceuticals Inc.
Figures




References
-
- Khan A, Vandeplas G, Huynh TMT, et al. The global allergy and asthma European network (GALEN) rhinosinusitis cohort: A large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57(1):32-42. - PubMed
-
- Kowalski ML, Agache I, Bavbek S, et al. Diagnosis and management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI position paper. Allergy. 2019;74(1):28-39. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

