Non-Chinese herbal medicines for functional dyspepsia
- PMID: 37323050
- PMCID: PMC10267606
- DOI: 10.1002/14651858.CD013323.pub2
Non-Chinese herbal medicines for functional dyspepsia
Abstract
Background: One-third of people with gastrointestinal disorders, including functional dyspepsia, use some form of complementary and alternative medicine, including herbal medicines.
Objectives: The primary objective is to assess the effects of non-Chinese herbal medicines for the treatment of people with functional dyspepsia.
Search methods: We searched the following electronic databases on 22 December 2022: Cochrane Central Register of Controlled Trials, MEDLINE, Embase, Allied and Complementary Medicine Database, Latin American and Caribbean Health Sciences Literature, among other sources, without placing language restrictions.
Selection criteria: We included RCTs comparing non-Chinese herbal medicines versus placebo or other treatments in people with functional dyspepsia.
Data collection and analysis: Two review authors independently screened references, extracted data and assessed the risk of bias from trial reports. We used a random-effects model to calculate risk ratios (RRs) and mean differences (MDs). We created effect direction plots when meta-analysis was not possible, following the reporting guideline for Synthesis without Meta-analysis (SWiM). We used GRADE to assess the certainty of the evidence (CoE) for all outcomes.
Main results: We included 41 trials with 4477 participants that assessed 27 herbal medicines. This review evaluated global symptoms of functional dyspepsia, adverse events and quality of life; however, some studies did not report these outcomes. STW5 (Iberogast) may moderately improve global symptoms of dyspepsia compared with placebo at 28 to 56 days; however, the evidence is very uncertain (MD -2.64, 95% CI -4.39 to -0.90; I2 = 87%; 5 studies, 814 participants; very low CoE). STW5 may also increase the improvement rate compared to placebo at four to eight weeks' follow-up (RR 1.55, 95% CI 0.98 to 2.47; 2 studies, 324 participants; low CoE). There was little to no difference in adverse events for STW5 compared to placebo (RR 0.92, 95% CI 0.52 to 1.64; I2 = 0%; 4 studies, 786 participants; low CoE). STW5 may cause little to no difference in quality of life compared to placebo (no numerical data available, low CoE). Peppermint and caraway oil probably result in a large improvement in global symptoms of dyspepsia compared to placebo at four weeks (SMD -0.87, 95% CI -1.15 to -0.58; I2 = 0%; 2 studies, 210 participants; moderate CoE) and increase the improvement rate of global symptoms of dyspepsia (RR 1.53, 95% CI 1.30 to 1.81; I2 = 0%; 3 studies, 305 participants; moderate CoE). There may be little to no difference in the rate of adverse events between this intervention and placebo (RR 1.56, 95% CI 0.69 to 3.53; I2 = 47%; 3 studies, 305 participants; low CoE). The intervention probably improves the quality of life (measured on the Nepean Dyspepsia Index) (MD -131.40, 95% CI -193.76 to -69.04; 1 study, 99 participants; moderate CoE). Curcuma longa probably results in a moderate improvement global symptoms of dyspepsia compared to placebo at four weeks (MD -3.33, 95% CI -5.84 to -0.81; I2 = 50%; 2 studies, 110 participants; moderate CoE) and may increase the improvement rate (RR 1.50, 95% CI 1.06 to 2.11; 1 study, 76 participants; low CoE). There is probably little to no difference in the rate of adverse events between this intervention and placebo (RR 1.26, 95% CI 0.51 to 3.08; 1 study, 89 participants; moderate CoE). The intervention probably improves the quality of life, measured on the EQ-5D (MD 0.05, 95% CI 0.01 to 0.09; 1 study, 89 participants; moderate CoE). We found evidence that the following herbal medicines may improve symptoms of dyspepsia compared to placebo: Lafonesia pacari (RR 1.52, 95% CI 1.08 to 2.14; 1 study, 97 participants; moderate CoE), Nigella sativa (SMD -1.59, 95% CI -2.13 to -1.05; 1 study, 70 participants; high CoE), artichoke (SMD -0.34, 95% CI -0.59 to -0.09; 1 study, 244 participants; low CoE), Boensenbergia rotunda (SMD -2.22, 95% CI -2.62 to -1.83; 1 study, 160 participants; low CoE), Pistacia lenticus (SMD -0.33, 95% CI -0.66 to -0.01; 1 study, 148 participants; low CoE), Enteroplant (SMD -1.09, 95% CI -1.40 to -0.77; 1 study, 198 participants; low CoE), Ferula asafoetida (SMD -1.51, 95% CI -2.20 to -0.83; 1 study, 43 participants; low CoE), ginger and artichoke (RR 1.64, 95% CI 1.27 to 2.13; 1 study, 126 participants; low CoE), Glycyrrhiza glaba (SMD -1.86, 95% CI -2.54 to -1.19; 1 study, 50 participants; moderate CoE), OLNP-06 (RR 3.80, 95% CI 1.70 to 8.51; 1 study, 48 participants; low CoE), red pepper (SMD -1.07, 95% CI -1.89 to -0.26; 1 study, 27 participants; low CoE), Cuadrania tricuspidata (SMD -1.19, 95% CI -1.66 to -0.72; 1 study, 83 participants; low CoE), jollab (SMD -1.22, 95% CI -1.59 to -0.85; 1 study, 133 participants; low CoE), Pimpinella anisum (SMD -2.30, 95% CI -2.79 to -1.80; 1 study, 107 participants; low CoE). The following may provide little to no difference compared to placebo: Mentha pulegium (SMD -0.38, 95% CI -0.78 to 0.02; 1 study, 100 participants; moderate CoE) and cinnamon oil (SMD 0.38, 95% CI -0.17 to 0.94; 1 study, 51 participants; low CoE); moreover, Mentha longifolia may increase dyspeptic symptoms (SMD 0.46, 95% CI 0.04 to 0.88; 1 study, 88 participants; low CoE). Almost all the studies reported little to no difference in the rate of adverse events compared to placebo except for red pepper, which may result in a higher risk of adverse events compared to placebo (RR 4.31, 95% CI 1.56 to 11.89; 1 study, 27 participants; low CoE). With respect to the quality of life, most studies did not report this outcome. When compared to other interventions, essential oils may improve global symptoms of dyspepsia compared to omeprazole. Peppermint oil/caraway oil, STW5, Nigella sativa and Curcuma longa may provide little to no benefit compared to other treatments.
Authors' conclusions: Based on moderate to very low-certainty evidence, we identified some herbal medicines that may be effective in improving symptoms of dyspepsia. Moreover, these interventions may not be associated with important adverse events. More high-quality trials are needed on herbal medicines, especially including participants with common gastrointestinal comorbidities.
배경: 기능성 소화불량을 포함한 위장 장애가 있는 사람의 1/3은 약초를 포함한 어떤 형태의 보완 및 대체 의학을 사용한다. 목적: 1차 목표는 기능성 소화불량 환자의 치료를 위한 비한약의 효과를 평가하는 것이다. 검색 전략: 2022년 12월 22일에 다음 전자 데이터베이스를 검색했다. Cochrane Central Register of Controlled Trials, MEDLINE, Embase, Allied and Complementary Medicine Database, Latin American and Caribbean Health Sciences Literature 등 다른 출처에서 언어제한 없이 검색했다. 선정 기준: 기능성 소화불량 환자를 대상으로 비한약과 위약 또는 기타 치료법을 비교하는 RCT를 포함했다. 자료 수집 및 분석: 2명의 검토 저자가 독립적으로 참고문헌을 선별하고 데이터를 추출했으며 임상시험 보고서의 비뚤림 위험을 평가했다. 무작위 효과 모델을 사용하여 위험 비율(RR)과 평균 차이(MD)를 계산했다. SWiM(Synthesis without Meta‐analysis)에 대한 보고 지침에 따라 메타 분석이 불가능할 때 효과 방향 도표를 만들었다. GRADE를 사용하여 모든 결과에 대한 근거의 확실성(CoE)을 평가했다. 주요 결과: 27개의 약초를 평가한 4477명의 참가자를 대상으로 한 41건의 임상시험을 포함했다. 이 검토에서는 기능성 소화불량의 전반적인 증상, 부작용 및 삶의 질을 평가했다. 그러나 일부 연구에서는 이러한 결과를 보고하지 않았다. STW5(이베로가스트)는 28일에서 56일 사이에 위약에 비해 전반적인 소화불량 증상을 적당히 개선할 수 있다. 그러나 근거는 매우 불확실하다(MD ‐2.64, 95% CI ‐4.39 ~ ‐0.90; I 2 = 87%; 5건의 연구, 814명의 참가자; 매우 낮은 CoE). STW5는 또한 4주에서 8주의 후속 조치에서 위약에 비해 개선률을 증가시킬 수 있다(RR 1.55, 95% CI 0.98~2.47; 2건의 연구, 324명의 참가자, 낮은 CoE). 위약과 비교하여 STW5에 대한 부작용의 차이는 거의 또는 전혀 없었다(RR 0.92, 95% CI 0.52 ~ 1.64; I 2 = 0%; 4건의 연구, 786명의 참가자, 낮은 CoE). STW5는 위약과 비교하여 삶의 질에 거의 또는 전혀 차이를 일으키지 않을 수 있다(사용 가능한 수치 데이터 없음, 낮은 CoE). 페퍼민트와 캐러웨이 오일은 4주째에 위약에 비해 소화불량의 전반적인 증상이 크게 개선될 가능성이 있으며(SMD ‐0.87, 95% CI ‐1.15 ~ ‐0.58; I 2 = 0%; 2건의 연구, 210명의 참가자, 중간 CoE), 소화불량의 전반적인 증상 개선률을 높였다(RR 1.53, 95% CI 1.30 ~ 1.81; I 2 = 0%; 3건의 연구, 305명의 참가자, 중간 CoE). 이 중재와 위약 사이의 부작용 비율에는 거의 또는 전혀 차이가 없을 수 있다(RR 1.56, 95% CI 0.69 ~ 3.53; I 2 = 47%; 3건의 연구, 305명의 참가자, 낮은 CoE). 중재는 아마도 삶의 질을 향상시킬 것이다(네피안 소화불량 지수로 측정)(MD ‐131.40, 95% CI ‐193.76 ~ ‐69.04; 1건의 연구, 99명의 참가자, 중간 정도의 CoE). Curcuma longa 는 아마도 4주에 위약과 비교하여 소화불량의 전반적인 증상을 약간 개선시켰을 것이며(MD ‐3.33, 95% CI ‐5.84 ~ ‐0.81; I 2 = 50%; 2건의 연구, 110명의 참가자; 중간 CoE) 개선률(RR 1.50, 95% CI 1.06~2.11, 1건의 연구, 76명의 참가자, 낮은 CoE)을 증가할 수 있다. 이 중재와 위약 사이의 부작용 비율에는 거의 차이가 없을 것이다(RR 1.26, 95% CI 0.51 ~ 3.08; 1건의 연구, 89명의 참가자, 중간 CoE). 중재는 아마도 EQ‐5D에서 측정된 삶의 질을 향상시킬 것이다(MD 0.05, 95% CI 0.01~0.09; 1건의 연구, 89명의 참가자; 중간 CoE). 다음 약초가 위약에 비해 소화불량 증상을 개선할 수 있다는 근거를 발견했다. Lafonesia pacari (RR 1.52, 95% CI 1.08~2.14; 1건의 연구, 97명의 참여자; 중간 CoE), Nigella sativa (SMD ‐1.59, 95% CI ‐2.13~‐1.05; 1건의 연구, 70명의 참여자; 높은 CoE), 아티초크 (SMD ‐0.34, 95% CI ‐0.59 ~ ‐0.09; 1건의 연구, 244명의 참여자; 낮은 CoE), Boensenbergia rotunda (SMD ‐2.22, 95% CI ‐2.62 ~ ‐1.83; 1건의 연구, 160명의 참여자; 낮은 CoE), Pistacia lenticus (SMD ‐0.33, 95% CI ‐0.66 ~ ‐0.01; 1건의 연구, 148명의 참가자; 낮은 CoE), Enteroplant(SMD ‐1.09, 95% CI ‐1.40 ~ ‐0.77; 1건의 연구, 198명의 참가자; 낮은 CoE) , Ferula asafoetida (SMD ‐1.51, 95% CI ‐2.20 ~ ‐0.83; 1건의 연구, 43명의 참가자; 낮은 CoE), 생강 및 아티초크(RR 1.64, 95% CI 1.27 ~ 2.13; 1건의 연구, 126명의 참가자; 낮은 CoE) , 감초 (SMD ‐1.86, 95% CI ‐2.54 ~ ‐1.19; 1건의 연구, 50명의 참가자; 중간 CoE), OLNP‐06(RR 3.80, 95% CI 1.70 ~ 8.51; 1건의 연구, 48명의 참가자; 낮은 CoE) , 고추(SMD ‐1.07, 95% CI ‐1.89 ~ ‐0.26; 1건의 연구, 27명의 참가자; 낮은 CoE), Cuadrania tricuspidata (SMD ‐1.19, 95% CI ‐1.66 ~ ‐0.72; 1건의 연구, 83명의 참가자; 낮은 CoE), jollab(SMD ‐1.22, 95% CI ‐1.59~‐0.85; 1건의 연구, 133명의 참가자; 낮은 CoE), Pimpinella ansum (SMD ‐2.30, 95% CI ‐2.79~‐1.80; 1건의 연구, 107명의 참가자 ; 낮은 CoE). 다음은 위약과 비교하여 차이가 거의 없거나 전혀 없을 수 있다. Mentha pulegium (SMD ‐0.38, 95% CI ‐0.78 ~ 0.02; 1건의 연구, 100명의 참가자; 중간 CoE) 및 계피유(SMD 0.38, 95% CI ‐0.17 ~ 0.94; 1건의 연구, 51명의 참가자; 낮은 CoE); 또한 Mentha longifolia 는 소화불량 증상을 증가시킬 수 있다(SMD 0.46, 95% CI 0.04~0.88; 1건의 연구, 88명의 참가자; 낮은 CoE). 거의 모든 연구에서 위약에 비해 이상 반응의 위험이 더 높을 수 있는 고추를 제외하고 위약에 비해 이상 반응 발생률의 차이가 거의 또는 전혀 없다고 보고했다(RR 4.31, 95% CI 1.56~11.89; 1개 연구, 참가자 27명, 낮은 CoE). 삶의 질과 관련하여 대부분의 연구에서는 이러한 결과를 보고하지 않았다. 다른 중재와 비교할 때 에센셜 오일은 오메프라졸에 비해 소화 불량의 전반적인 증상을 개선할 수 있다. 페퍼민트 오일/캐러웨이 오일, STW5, Nigella sativa 및 Curcuma longa 는 다른 치료법에 비해 이점이 거의 또는 전혀 없을 수 있다. 연구진 결론: 중간에서 매우 낮은 확실성 근거를 바탕으로 소화불량 증상 개선에 효과적일 수 있는 몇 가지 약초를 확인했다. 또한, 이러한 중재는 중요한 부작용과 관련이 없을 수 있다. 특히 일반적인 위장병 동반 질환이 있는 참가자를 포함하여 약초에 대해 더 높은 품질의 시험이 필요하다.
Antecedentes: Un tercio de las personas con trastornos gastrointestinales, incluida la dispepsia funcional, recurre a algún tipo de medicina complementaria y alternativa, incluidas las plantas medicinales.
Objetivos: El objetivo principal es evaluar los efectos de las plantas medicinales no chinas para el tratamiento de las personas con dispepsia funcional. MÉTODOS DE BÚSQUEDA: El 22 de diciembre de 2022 se realizaron búsquedas en las siguientes bases de datos electrónicas: Registro Cochrane central de ensayos controlados (Cochrane Central Register of Controlled Trials; CENTRAL), MEDLINE, Embase, Allied and Complementary Medicine Database, Latin American and Caribbean Health Sciences Literature, entre otras fuentes, sin poner restricciones de idioma. CRITERIOS DE SELECCIÓN: Se incluyeron los ECA que compararan plantas medicinales no chinas versus placebo u otros tratamientos en personas con dispepsia funcional. OBTENCIÓN Y ANÁLISIS DE LOS DATOS: Dos autores de la revisión examinaron de forma independiente las referencias, extrajeron los datos y evaluaron el riesgo de sesgo de los informes de los ensayos. Se utilizó un modelo de efectos aleatorios para calcular las razones de riesgos (RR) y las diferencias de medias (DM). Se crearon gráficos de la dirección del efecto cuando no fue posible realizar un metanálisis, siguiendo las guías de presentación de informes para la síntesis sin metanálisis. Se utilizó el método GRADE para evaluar la certeza de la evidencia de todos los desenlaces.
Resultados principales: Se incluyeron 41 ensayos con 4477 participantes que evaluaron 27 plantas medicinales. Esta revisión evaluó los síntomas generales de la dispepsia funcional, los eventos adversos y la calidad de vida; sin embargo, algunos estudios no informaron sobre estos desenlaces. El STW5 (Iberogast) podría mejorar moderadamente los síntomas generales de dispepsia en comparación con placebo a los 28 a 56 días; sin embargo, la evidencia es muy incierta (DM ‐2,64; IC del 95%: ‐4,39 a ‐0,90; I 2 = 87%; cinco estudios, 814 participantes; evidencia de certeza muy baja). El STW5 también podría aumentar la tasa de mejoría en comparación con el placebo a las cuatro u ocho semanas de seguimiento (RR 1,55; IC del 95%: 0,98 a 2,47; dos estudios, 324 participantes; evidencia de certeza baja). Hubo poca o ninguna diferencia en los eventos adversos para el STW5 en comparación con placebo (RR 0,92; IC del 95%: 0,52 a 1,64; I 2 = 0%; cuatro estudios, 786 participantes; evidencia de certeza baja). El STW5 podría dar lugar a poca o ninguna diferencia en la calidad de vida en comparación con placebo (no hay datos numéricos disponibles, evidencia de certeza baja). El aceite de menta y de comino probablemente dan lugar a una gran mejoría de los síntomas generales de dispepsia en comparación con el placebo a las cuatro semanas (DME ‐0,87; IC del 95%: ‐1,15 a ‐0,58; I 2 = 0%; dos estudios, 210 participantes; evidencia de certeza moderada) y aumentan la tasa de mejoría de los síntomas generales de dispepsia (RR 1,53; IC del 95%: 1,30 a 1,81; I 2 = 0%; tres estudios, 305 participantes; evidencia de certeza moderada). Podría haber poca o ninguna diferencia en la tasa de eventos adversos entre esta intervención y el placebo (RR 1,56; IC del 95%: 0,69 a 3,53; I 2 = 47%; tres estudios, 305 participantes; evidencia de certeza baja). La intervención probablemente mejora la calidad de vida (medida con el Nepean Dyspepsia Index) (DM ‐131,40; IC del 95%: ‐193,76 a ‐69,04; un estudio, 99 participantes; evidencia de certeza moderada). La Curcuma longa probablemente produce una mejoría moderada de los síntomas generales de dispepsia en comparación con placebo a las cuatro semanas (DM ‐3,33; IC del 95%: ‐5,84 a ‐0,81; I 2 = 50%; dos estudios, 110 participantes; evidencia de certeza moderada) y podría aumentar la tasa de mejoría (RR 1,50; IC del 95%: 1,06 a 2,11; un estudio, 76 participantes; evidencia de certeza baja). Probablemente haya poca o ninguna diferencia en la tasa de eventos adversos entre esta intervención y el placebo (RR 1,26; IC del 95%: 0,51 a 3,08; un estudio, 89 participantes; evidencia de certeza moderada). La intervención probablemente mejora la calidad de vida, medida en el EQ‐5D (DM 0,05; IC del 95%: 0,01 a 0,09; un estudio, 89 participantes; evidencia de certeza moderada). Se encontró evidencia de que las siguientes plantas medicinales podrían mejorar los síntomas de dispepsia en comparación con placebo: Lafonesia pacari (RR 1,52; IC del 95%: 1,08 a 2,14; un estudio, 97 participantes; evidencia de certeza moderada), Nigella sativa (DME ‐1,59; IC del 95%: ‐2,13 a ‐1,05; un estudio, 70 participantes; evidencia de certeza alta), alcachofa (DME ‐0,34; IC del 95%: ‐0,59 a ‐0,09; un estudio, 244 participantes; evidencia de certeza baja), Boensenbergia rotunda (DME ‐2,22; IC del 95%: ‐2,62 a ‐1,83; un estudio: 160 participantes; evidencia de certeza baja), Pistacia lenticus (DME ‐0,33; IC del 95%: ‐0,66 a ‐0,01; un estudio: 148 participantes; evidencia de certeza baja), Enteroplant (DME ‐1,09; IC del 95%: ‐1,40 a ‐0,77; un estudio: 198 participantes; evidencia de certeza baja), Ferula asafoetida (DME ‐1,51; IC del 95%: ‐2,20 a ‐0,83; un estudio: 43 participantes; evidencia de certeza baja), jengibre y alcachofa (RR 1,64; IC del 95%: 1,27 a 2,13; un estudio, 126 participantes; evidencia de certeza baja), Glycyrrhiza glaba (DME ‐1,86; IC del 95%: ‐2,54 a ‐1,19; un estudio, 50 participantes; evidencia de certeza moderada), OLNP‐06 (RR 3,80; IC del 95%: 1,70 a 8,51; un estudio, 48 participantes; evidencia de certeza baja), pimiento rojo (DME ‐1,07; IC del 95%: ‐1,89 a ‐0,26; un estudio, 27 participantes; evidencia de certeza baja), Cuadrania tricuspidata (DME ‐1,19; IC del 95%: ‐1,66 a ‐0,72; un estudio, 83 participantes; evidencia de certeza baja), jollab (DME ‐1,22; IC del 95%: ‐1,59 a ‐0,85; un estudio, 133 participantes; evidencia de certeza baja), Pimpinella anisum (DME ‐2,30; IC del 95%: ‐2,79 a ‐1,80; un estudio, 107 participantes; evidencia de certeza baja). Las siguientes podrían dar lugar a poca o ninguna diferencia en comparación con placebo: Mentha pulegium (DME ‐0,38; IC del 95%: ‐0,78 a 0,02; un estudio, 100 participantes; evidencia de certeza moderada) y aceite de canela (DME 0,38; IC del 95%: ‐0,17 a 0,94; un estudio, 51 participantes; evidencia de certeza baja); además, Mentha longifolia podría aumentar los síntomas dispépticos (DME 0,46; IC del 95%: 0,04 a 0,88; un estudio, 88 participantes; evidencia de certeza baja). Casi todos los estudios informaron poca o ninguna diferencia en la tasa de eventos adversos en comparación con placebo, excepto en el caso de la pimienta roja, que podría dar lugar a un mayor riesgo de eventos adversos en comparación con placebo (RR 4,31; IC del 95%: 1,56 a 11,89; un estudio, 27 participantes; evidencia de certeza baja). Con respecto a la calidad de vida, la mayoría de los estudios no informaron acerca de este desenlace. En comparación con otras intervenciones, los aceites esenciales podrían mejorar los síntomas generales de la dispepsia en comparación con el omeprazol. El aceite de menta/aceite de comino, el STW5, la Nigella sativa y la Curcuma longa podrían aportar poco o ningún beneficio en comparación con otros tratamientos.
Conclusiones de los autores: Sobre la base de evidencia de certeza moderada a muy baja, se identificaron algunas plantas medicinales que podrían ser eficaces para mejorar los síntomas de la dispepsia. Además, es posible que estas intervenciones no se asocien con eventos adversos importantes. Se necesitan más ensayos de calidad alta sobre las plantas medicinales, especialmente con participantes con comorbilidades gastrointestinales frecuentes.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
GB: none known CV: none known MA: none known CP: none known JVAF: none known. JVAF is a managing editor for the Cochrane Metabolic and Endocrine Disorders Group and a contact editor for the Urology Group. He was not involved in the editorial process of this manuscript.
Figures











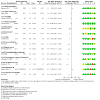







Comment in
-
Herbal Medicines for Functional Dyspepsia.Am Fam Physician. 2024 Apr;109(4):313-314. Am Fam Physician. 2024. PMID: 38648828 No abstract available.
References
References to studies included in this review
Alizadeh‐Naini 2020 {published data only}
-
- Alizadeh-Naini M, Yousefnejad H, Hejazi N. The beneficial health effects of Nigella sativa on Helicobacter pylori eradication, dyspepsia symptoms, and quality of life in infected patients: A pilot study. Phytotherapy Research 2020;34(6):1367-76. - PubMed
Allan 2012 {published data only}
-
- Allan JJ, Raveendra KR, Jayachandra Srinivasa V, Sushma KR, Goudar KS, Shivaprasad HN, et al. An extract of Glycyrrhiza glabra (GutGard) alleviates symptoms of functional dyspepsia: A randomized, double-blind, placebo-controlled study. Evid Based Complement Alternat Med 2012;2012:216970. [DOI: 10.1155/2012/216970] - DOI - PMC - PubMed
Azimi 2017 {published data only}
-
- Azimi M, Zahedi MJ, Mehrabani M, Tajadini H, Zolala F, Baneshi MR, et al. Effects of Iranian traditional medicine remedies (Apium Graveolence and Trachyspermum Copticum) on modifying the quality of life in patients with functional dyspepsia: A double- blind randomized clinical trial. Galen Medical Journal 2017;6(2):102-9.
Babaeian 2017 {published data only}
-
- Babaeian M Naseri M Kamalinejad M Ghaffari F Emadi F Feizi A Rafiei R Mazaheri M Hasheminejad SA Park J-W Adibi P. The efficacy of Mentha longifolia in the treatment of patients with postprandial distress syndrome: a double-blind, randomized clinical trial. Iranian Red Crescent Medical Journal 2017;19(2):1-10.
Bordbar 2020 {published data only}
Bortolotti 2002 {published data only}
-
- Bortolotti M, Coccia G, Grossi G, Miglioli M. The treatment of functional dyspepsia with red pepper. Alimentary Pharmacology and Therapeutics 2002;16(6):1075-82. - PubMed
Braden 2009 {published data only}
-
- Braden B, Caspary W, Borner N, Vinson B, Schneider ARJ. Clinical effects of STW 5 (Iberogast) are not based on acceleration of gastric emptying in patients with functional dyspepsia and gastroparesis. Neurogastroenterology and Motility 2009;21(6):632-e25. - PubMed
Chey 2019 {published data only}
-
- Chey WD, Lacy BE, Cash BD, Epstein M, Shah SM. Efficacy of caraway oil/L-menthol plus usual care vs placebo plus usual care, in functional dyspepsia patients with post-prandial distress (PDS) or epigastric pain (EPS) syndromes: Results from a US RCT. Gastroenterology 2017;152(5 Supplement 1):S307.
-
- Lacy BE, Chey WD, Cash BD, Epstein M, Shah SM. A caraway oil/menthol combination improves functional dyspepsia (FD) symptoms within the first 24 hours: Results of a randomized controlled trial, which allowed usual FD treatments. Gastroenterology 2017;152(5 Supplement 1):S307.
Chitapanarux 2020 {published data only}
-
- Chitapanarux T Lertprasertsuke N Toworakul C. Efficacy and Safety of Fingerroot (Boesenbergia rotunda) Extract in Patients with Functional Dyspepsia: a Randomized, Placebo-Controlled Trial. Digestion 2020;102(4):599-606. - PubMed
-
- TCTR20170816002. Efficacy and safety of Boesenbergia rotunda in functional dyspepsia patients: a randomized controlled trial. Digestion 2017;102(4):599-606. - PubMed
Dabos 2010 {published data only}
-
- Dabos KJ, Sfika E, Vlatta LJ, Frantzi D, Amygdalos GI, Giannikopoulos G. Is Chios mastic gum effective in the treatment of functional dyspepsia? A prospective randomised double-blind placebo controlled trial. Journal of Ethnopharmacology 2010;127(2):205-9. - PubMed
Da Mota Menezes 2006 {published data only}
-
- Da Mota Menezes V, Atallah AN, Lapa AJ, Catapani WR. Assessing the therapeutic use of Lafoensia pacari St. Hil. extract (Mangava-Brava) in the eradication of Helicobacter pylori: Double-blind randomized clinical trial. Helicobacter 2006;11(3):188-95. - PubMed
-
- da Mota Menezes, Valfredo. Avaliação do uso terapêutico do extrato de Lafoensia pacari St. Hil. Mangava-Brava na erradicação do Helicobacter pylori: Ensaio Clínico Randomizado Duplo Cego. https://repositorio.unifesp.br/handle/11600/8984 2006-04-03.
Eftekharafzali 2018 {published data only}
-
- Eftekharafzali M, Mehrabani M, Tajadini H, Ahmadi B, Zahedi MJ. Effect of "pistacia atlantica" resin (Baneh) on functional dyspepsia: A double-blind, randomized clinical study. Iranian Red Crescent Medical Journal 2018;20(7):e63822.
Ghoshegir 2015 {published data only}
Giacosa 2015 {published data only}
-
- Giacosa A Guido D Grassi M Riva A Morazzoni P Bombardelli E Perna S Faliva MA Rondanelli M. The effect of ginger (Zingiber officinalis) and artichoke (Cynara cardunculus) extract supplementation on functional dyspepsia: A randomised, double-blind, and placebo-controlled clinical trial. Evid Based Complement Alternat Med 2015;2015:915087. - PMC - PubMed
Holtmann 2003 {published data only}
-
- Holtmann G, Adam B, Haag S, Collet W, Grunewald E, Windeck T. Efficacy of artichoke leaf extract in the treatment of patients with functional dyspepsia: A six-week placebo-controlled, double-blind, multicentre trial. Alimentary Pharmacology and Therapeutics 2003;18(11-12):1099-105. - PubMed
Holtmann 2013 {published data only}
-
- Holtmann G, Andrews J, Holloway R, Beilby J, Adam B, Pilichiewicz AN, et al. Symptom improvement during treatment is linked to alterations of sensory function in functional dyspepsia: A placebo controlled randomised treatment trial. Journal of Gastroenterology and Hepatology 2013;28((Talley) University of Newcastle, Newcastle, NSW, Australia):126-7.
-
- Holtmann G, Andrews JM, Holloway RH, Beilby J, Adam B, Pilichiewicz AN, et al. A placebo controlled randomised treatment trial for functional dyspepsia including post-treatment drug withdraw and placebo withdraw effects. Gastroenterology 2013;144(5 SUPPL. 1):S733.
-
- Pilichiewicz AN, Andrews JM, Goess C, McMahon J, Barrett B, Fraser RJ, et al. Placebo effects in functional dyspepsia: A preliminary analysis on symptoms in an ongoing randomized placebo controlled trial. Gastroenterology 2012;142(5 SUPPL. 1):S469.
-
- Pilichiewicz AN, Andrews JM, Goess C, Mcmahon J, Fraser RJ, Beilby J, et al. A preliminary analysis of the effect Iberogast, Nexium, dual Iberogast + Nexium or dual placebo on symptoms in patients with functional dyspepsia. Journal of Gastroenterology and Hepatology 2011;26((Talley) Faculty of Health, University of Newcastle, Newcastle, Australia):85.
Hu 2011 {published data only}
Khonche 2017 {published data only}
-
- Khonche A, Fallah Huseini H, abdi H, Mohtashami R, Nabati F, Kianbakht S. Efficacy of Mentha pulegium extract in the treatment of functional dyspepsia: A randomized double-blind placebo-controlled clinical trial. Journal of Ethnopharmacology 2017;206((Mohtashami) Medicine Quran and Hadith Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran, Islamic Republic of):267-73. - PubMed
Kupcinskas 2008 {published data only}
-
- Kupcinskas L, Lafolie P, Lignell A, Kiudelis G, Jonaitis L, Adamonis K, et al. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: A prospective, randomized, double blind, and placebo-controlled study. Phytomedicine 2008;15(6-7):391-9. - PubMed
Madisch 1999 {published data only}
-
- Madisch A, Heydenreich C-J, Wieland V, Hufnagel R, Hotz J. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride: A multicenter, reference-controlled double-blind equivalence study. Arzneimittel-Forschung/Drug Research 1999;49(11):925-32. - PubMed
Madisch 2001a {published data only}
-
- Madisch A, Melderis H, Mayr G, Sassin I, Hotz J. Commercially available herbal preparation and its modified dispense in patients with functional dyspepsia. Results of a double-blind, placebo-controlled, randomized multicenter trial. Zeitschrift fur Gastroenterologie 2001;39(7):511-7. - PubMed
Madisch 2004 {published data only}
-
- Madisch A, Holtmann G, Mayr G, Vinson B, Hotz J. Treatment of functional dyspepsia with a herbal preparation: A double-blind, randomized, placebo-controlled, multicenter trial. Digestion 2004;69(1):45-52. - PubMed
Mala 2018 {published data only}
May 2000 {published data only}
-
- May B, Kohler S, Schneider B. Efficacy and tolerability of a fixed combination of peppermint oil and caraway oil in patients suffering from functional dyspepsia. Alimentary Pharmacology and Therapeutics 2000;14(12):1671-7. - PubMed
Mohtashami 2015 {published data only}
-
- Mohtashami R, Fallah Huseini H, Heydari M, Amini M, Sadeqhi Z, Ghaznavi H, et al. Efficacy and safety of honey based formulation of Nigella sativa seed oil in functional dyspepsia: A double blind randomized controlled clinical trial. Journal of Ethnopharmacology 2015;175((Mehrzadi) Department of Pharmacology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran, Islamic Republic of):147-52. - PubMed
Panda 2020 {published data only}
-
- Panda M SK, Nirvanashetty S, Parachur VA, Krishnamoorthy C, Dey S. A Randomized, Double-Blind, Placebo Controlled, Parallel-Group, Comparative Clinical Study to Evaluate the Efficacy and Safety of OLNP-06 versus Placebo in Subjects with Functional Dyspepsia. Journal of Dietary Supplements 2020;19(8):226-237. [DOI: 10.1080/19390211.2020.1856996] - DOI - PubMed
Pasalar 2015 {published data only}
-
- Pasalar M, Choopani R, Mosaddegh M, Kamalinejad M, Mohagheghzadeh A, Fattahi MR, et al. Efficacy and safety of jollab to treat functional dyspepsia: A randomized placebo-controlled clinical trial. Explore: The Journal of Science and Healing 2015;11(3):199-207. - PubMed
-
- Ranjbar, M, Pasalar, M, Lankarani, KB, Choopani, R, Rezaeizadeh, H. A traditional Persian medicine beverage could improve quality of life in dyspeptic patients. Journal of medicinal plant and natural product research 2016;82(S1):S1-S381. [DOI: 10.1055/s-0036-1596992] - DOI
Puttapitakpong 2016 {published data only}
-
- Puttapitakpong C, Jearjesdakul J. Effectiveness of curcuma longa linn compared with omeprazole on treatment of functional dyspepsia. Gastroenterology 2016;150(4 SUPPL. 1):S43.
Rafieian‐Kopaei 2005 {published data only}
-
- Rafieian-Kopaei M, Hosseini-Asl K. Effects of Ocimum basilicum on functional dyspepsia: A double-blind placebo-controlled study. Iranian Journal of Medical Sciences 2005;30(3):134-7.
Rich 2017 {published data only}
-
- Holtmann G, Rich G, Wieland V, Funk P, Kieser M, Koehler S. Menthacarin for the treatment of epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS): Data of a placebocontrolled trial revisited. Zeitschrift fur Gastroenterologie 2015;53(8):KG253.
-
- Holtmann G, Stracke B. Effects of Menthacarin on symptoms and quality of life in patients with functional dyspepsia: Result of a 8-week optional placebo controlled follow-up. Zeitschrift fur Gastroenterologie 2016;54(8):KV420.
-
- Holtmann GJ, Stracke B. Sustained treatment effects of menthacarin on symptoms and quality of life in patients with functional dyspepsia 8 weeks after the end of a 4-week placebo-controlled trial. United European Gastroenterology Journal 2017;5(5 Supplement 1):A790-1.
-
- Rich G, Shah A, Koloski N, Funk P, Stracke B, Kohler S, Holtmann G. A randomized placebo-controlled trial on the effects of Menthacarin, a proprietary peppermint- and caraway-oil-preparation, on symptoms and quality of life in patients with functional dyspepsia. Neurogastroenterology and Motility 2017;29(11):1-9. - PubMed
Rosch 2002 {published data only}
-
- Rosch W, Vinson B, Sassin I. A randomised clinical trial comparing the efficacy of a herbal preparation STW 5 with the prokinetic drug cisapride in patients with dysmotility type of functional dyspepsia. Zeitschrift fur Gastroenterologie 2002;40(6):401-8. - PubMed
Salem 2010 {published data only}
Sastry 2016 {published data only}
-
- CTRI/2017/08/009227. Pudin Hara Pearls & Pudin Hara Liquid evaluted for its efficacy & safety gor indication in Indigestion. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/08/009227 2017.
-
- Sastry JLN, Pandey SD, Vats A, Vedula A, Kumar S. Symptomatic management of functional dyspepsia: evaluation of efficacy and safety of pudin hara pearls and pudin hara liquid. International Journal Research in Ayurveda and Pharmacy 2016;7(3):65-9.
Sattarzadeh 2021 {published data only}
-
- Sattarzadeh MB, Asie S, Mohssen N, Mehri A, Foroogh A, Mohammad T, et al. Topical Mastic Oil for Treatment of Functional Dyspepsia: A Randomized Triple-Blind Controlled Trial. GMJ 2021-02-04;10:e1965.. [DOI: 10.31661/gmj.v10i0.1965] - DOI
Shin 2021 {published data only}
-
- A 8 week, Multi-center, Randomized, Double-blind, Placebo-Controlled Clinical Trial for the Evaluation of the Efficacy and Safety of CTE on Stomach Symptom. http://cris.nih.go.kr/cris/en/search/search_result_st01.jsp?seq=16298 First Submitted Date: 2020/03/24.
-
- Shin J, Oh TH, Kim JY, Shim JJ, Lee JL. Efficacy and Safety of the Cudrania tricuspidata Extract on Functional Dyspepsia: A Randomized Double-Blind Placebo-Controlled Multicenter Study. Journal of clinical medicine Published 2021 Nov 16.;10((22)):5323. [DOI: 10.3390/jcm10225323] - DOI - PMC - PubMed
Sun 2016 {published data only}
-
- Sun X, Ke M, Wang Z, Lin L, Zhong H, Zhang S, et al. Treatment of functional dyspepsia with enteroplant: A double-blind randomized placebo-controlled pilot phase II clinical trial. Neurogastroenterology and Motility 2014;26((Zeng, Tang) GI, Affiliated Hospital, Hainan Medical University, China):76.
Thamlikitkul 1989 {published data only}
-
- Thamlikitkul V, Bunyapraphatsara N, Dechatiwongse T, Theerapong S, Chantrakul C, Thanaveerasuwan T, et al. Randomized double blind study of Curcuma domestica Val. for dyspepsia. Journal of the Medical Association of Thailand = Chotmaihet thangphaet 1989;72(11):613-620. - PubMed
Vinson 2020 {published data only}
-
- Vinson B, Fink C, Wargenau M, Talley NJ, Holtmann GJ. 330: Efficacy and safety of STW-5-II in patients with functional dyspepsia: results from a double-blind, randomized, placebo-controlled, 8-week multicenter trial including a post-hoc analysis for patients meeting functional dyspepsia according to ROME IV. In: Gastroenterology. Vol. 162. 2022:S69.
-
- Vinson, B, Holtmann, G. Efficacy and safety of STW 5 II in patients with functional dyspepsia: results from a double-blind, randomized, multicenter trial. In: Zeitschrift für Gastroenterologie. 08 -9-2020. [DOI: 10.1055/s-0040-1716211] - DOI
Von Arnim 2007 {published data only}
-
- Von Arnim U, Peitz U, Vinson B, Gundermann K-J, Malfertheiner P. STW 5, a phytopharmacon for patients with functional dyspepsia: Results of a multicenter, placebo-controlled double-blind study. American Journal of Gastroenterology 2007;102(6):1268-1275. - PubMed
Yongwatana 2022 {published data only}
-
- Yongwatana K Harinwan K Chirapongsathorn S Opuchar K Sanpajit T Piyanirun W Puttapitakpong C. CURCUMA LONGA LINN VERSUS OMEPRAZOLE IN TREATMENT OF FUNCTIONAL DYSPEPSIA, A RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED TRIAL. The 35th Annual Meeting The Royal College of Physicians of Thailand 2019;156(6):S-. - PubMed
-
- Yongwatana K, Harinwan K, Chirapongsathorn S, Opuchar K, Sanpajit T, Piyanirun W, Puttapitakpong C. Curcuma longa Linn versus omeprazole in treatment of functional dyspepsia: A randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol 2022;37(2):335-341. [DOI: 10.1111/jgh.15705] [PMID: ] - DOI - PubMed
Zobeiri 2021 {published data only}
-
- Zobeiri M, Parvizi F, Shahpiri Z, Heydarpour F, Pourfarzam M, Memarzadeh MR, Rahimi R, Farzaei MH. Evaluation of the effectiveness of cinnamon oil soft capsule in patients with functional dyspepsia: a randomized double-blind placebo-controlled clinical trial. Evidence-Based Complementary andAlternative Medicine 2021;2021:1741-427. [DOI: 10.1155/2021/6634115] - DOI - PMC - PubMed
References to studies excluded from this review
ACTRN12619001236189 {published data only}
-
- ACTRN12619001236189. Curcumin for the Treatment of Digestive Complaints in Adults. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=378161 Date registered: 6/09/2019.
ACTRN12621000116820 {published data only}
-
- ACTRN12621000116820. The effects of the herbal preparation, STW5-II, on gastrointestinal motility in healthy volunteers. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=380779&... Date registered: 5/02/2021.
Arai 2011 {published data only}
-
- Arai Makoto, Matsumura Tomoaki, Yoshikawa Masaharu, Imazeki Fumio, Yokosuka Osamu. [Analysis of the Rikkunshito efficacy on patients with functional dyspepsia]. Nihon Yakurigaku Zasshi 2011;137(1):18-21. - PubMed
Arai 2012 {published data only}
-
- Arai M, Matsumura T, Tsuchiya N, Sadakane C, Inami R, Suzuki T, et al. Rikkunshito improves the symptoms in patients with functional dyspepsia, accompanied by an increase in the level of plasma ghrelin. Hepato-Gastroenterology 2012;59(113):62-66. - PubMed
Asha 2017 {published data only}
-
- Asha MK, Debraj D, Dethe S, Bhaskar A, Muruganantham N, Deepak M. Effect of flavonoid-rich extract of Glycyrrhiza glabra on gut-friendly microorganisms, commercial probiotic preparations, and digestive enzymes. Journal of Dietary Supplements 2017;14(1-3):323-333. - PubMed
Asif 2015 {published data only}
-
- Asif HM, Zaidi SF, Sugiyama T, Akhtar N, Usmanghani K. Phytomedicine-based and Quadruple Therapies in Helicobacter pylori Infection: A Comparative, Randomized Trial. Alternative Therapies in Health and Medicine 2015;21:33-39. - PubMed
Aydin 1997 {published data only}
-
- Aydin A, Ersoz G, Tekesin O, Akcicek E, Tuncyurek M, Batur Y. Does garlic oil have a role in the treatment of Helicobacter pylori infection? Turkish Journal of Gastroenterology 1997;8(2):181-184.
Barnick 1990 {published data only}
-
- Barnick CGW, Cardozo LD. The treatment of abdominal distension and dyspepsia with enteric coated peppermit oil following routine gynaecological intraperitoneal surgery. Journal of Obstetrics and Gynaecology 1990;10(5):423-424.
Bekar 2011 {published data only}
-
- Bekar O, Yilmaz Y, Gulten M. Kefir improves the efficacy and tolerability of triple therapy in eradicating Helicobacter pylori. Journal of Medicinal Food 2011;14(4):344-347. - PubMed
Bommer 2013 {published data only}
-
- Bommer S, Klein P, Suter A. A multicentre open clinical trial to assess the tolerability and efficacy of Boldocynara, a traditional herbal preparation for functional digestive disorders. Planta Medica 2013;79(13):..
Choi 2011 {published data only}
-
- Choi M-G, Rhee P-L, Park H, Lee OY, Lee KJ, Choi SC, et al. Randomized, controlled, multi-center trial comparing the safety and efficacy of DA-9701 and itopride hydrochloride in patients with functional dyspepsia: A non-inferiority trial. Journal of Neurogastroenterology and Motility 2011;21(3):414-422. - PMC - PubMed
Choi 2020 {published data only}
-
- NCT04008901. The Efficacy and Safety of "BST104" (Lonicera Flos Extract) in Mild to Moderate Functional Dyspepsia Subjects. https://clinicaltrials.gov/ct2/show/NCT04008901 2019.
CTRI/2022/03/041419 {published data only}
-
- CTRI/2022/03/041419. Efficacy of Pistacia Lenticus Plant (Rumi Mastagi) in Comparison to Levosupiride in Patients with Diabetic Gastroparesis A Double Blind Randomized Control Trial Study. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=66041 Date of registration: 28-03-2022. - PMC - PubMed
Di 2007 {published data only}
-
- Di Mario F, Cavallaro LG, Nouvenne A, Stefani N, Cavestro GM, Iori V, et al. A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: Something to learn from failure? Helicobacter 2007;12(3):238-243. - PubMed
Dimitrov 1983 {published data only}
-
- Dimitrov B, Mendizova A, Brailski K, Ivanov L, Bozhiianov V. Clinical trial of the preparation Verbascan in treating gastrointestinal and biliary diseases. Vŭtreshni bolesti 1983;22(1):18-24. - PubMed
Dos 2010 {published data only}
-
- Dos Santos SB, De Lima ACA, Melo ARDS, Frazao CDS, Cherpak GL. Comparision of the efficacy of oral mastic (Schinus terebinthiflolius Radd) with omeprazole in patients with gastritis and dyspeptic symptons: A randomized, double-blind. GED: Gastroenterologia Endoscopia Digestiva 2010;29(4):118-25.
Du 2014 {published data only}
Eady 2019 {published data only}
Fuhrer 2011 {published data only}
-
- Fuhrer M, Vogelsang H, Hammer J. A placebo controlled trial of an oral capsaicin load in patients with functional dyspepsia. Gastroenterology 2011;140(5 SUPPL. 1):S802. - PubMed
-
- Fuhrer M, Vogelsang H, Hammer J. A placebo-controlled trial of an oral capsaicin load in patients with functional dyspepsia. Neurogastroenterology and Motility 2011;23(10):918-e397. - PubMed
Gao 2007 {published data only}
-
- Gao LM, Yao SK, Zhang RX. Effect of Qingre Liqi Granule on clinical therapeutic efficacy, electrogastrogram and gastric emptying in patients with functional dyspepsia. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi 2007;27(6):505-508. - PubMed
Gasbarrini 2010 {published data only}
-
- Gasbarrini G, Zaccone V, Covino M, Gallo A. Effectiveness of a "cold dessert", with or without the addition of a mixture of digestive herbs, in subjects with "functional dyspepsia". Journal of Biological Regulators and Homeostatic Agents 2010;24(1):93-98. - PubMed
Gong 2019 {published data only}
-
- Gong Y, Huang X, Chen M, Xiong L. Teprenone improves gastric mucosal injury and dyspeptic symptoms in long-term nonsteroidal anti-inflammatory drug users. Journal of Gastroenterology and Hepatology 2019;34(8):1344-1350. - PubMed
Guo 2011 {published data only}
-
- Guo LK, Zhang CX, Guo XF. [Long-term efficacy and safety research on functional dyspepsia treated with electroacupuncture and Zhizhu Kuanzhong capsule]. Zhongguo Zhen Jiu 2011;31(12):1071-1077. - PubMed
Guo 2014 {published data only}
-
- Guo S-Y, Zeng Z-J. Jianpi Hewei decoction combined with Jianwei Xiaoshi oral liquid for treatment of functional dyspepsia. World Chinese Journal of Digestology 2014;22(25):3821-3825.
Hajiaghamohammadi 2016 {published data only}
Hashem‐Dabaghian 2016 {published data only}
IRCT20110310006026N9 {published data only}
-
- IRCT20110310006026N9. Evaluating the effects of standardized hydroalcoholic extract of marmotti (Salvia mirzayanii Rech. f. & Esfand) capsule as adjunctive treatment in patient with helicobacter pylori infection. https://en.irct.ir/trial/41703 2019;.:..
IRCT2017022332738N1 {published data only}
-
- IRCT2017022332738N1. Effect of Nardostachys jatamansi DC on clinical symptoms of functional dyspepcia /postprandial distress syndrome. https://en.irct.ir/trial/25428 2017;.:..
IRCT20170317033107N3 {published data only}
-
- IRCT20170317033107N3. Effectiveness of Persian herbal formulation on migraine associated with functional dyspepsia. https://en.irct.ir/trial/25626 2020.
IRCT20200128046288N3 {published data only}
-
- IRCT20200128046288N3. The Study of Combinatory effect of Cydonia oblanga, Cinnamomum zeylanicom, Pistacia lentiscus and Honey in Eradication of Gastric Helicobacter pylori Infection. https://www.irct.ir/trial/49741 Registration date: 2020-07-24.
Ivashkin 2022 {published data only}
-
- Ivashkin VT, Kudryavtseva AV, Krasnov GS, Poluektov YM, Morozova MA et al. Efficacy and safety of a food supplement with standardized menthol, limonene, and gingerol content in patients with irritable bowel syndrome: A double-blind, randomized, placebo-controlled trial. PLoS One 2022;17(6):e0263880. [DOI: 10.1371/journal.pone.0263880] - DOI - PMC - PubMed
Kammerer 2001 {published data only}
-
- Kammerer E, Fintelmann V. Curcuma-Wurzelstock bei dyspeptischen Beschwerden - Ergebnisse einer Anwendungsbeobachtung. Natura Med 2001;16(8):18-26.
KCT0003761 2019 {published data only}
-
- KCT0003761. Efficacy and safety study of WSY-S. https://cris.nih.go.kr/cris/search/detailSearch.do?seq=13532&search_... 2019;.:..
KCT0004085 2019 {published data only}
-
- KCT0004085. Study for the efficacy and safety of "BST104" in mild to moderate functional dyspepsia subjects. http://www.who.int/trialsearch/Trial2.aspx?TrialID=KCT0004085, 2019 2019;.:..
KCT0005265 2020 {published data only}
-
- KCT0005265. A Clinical Trial for the evaluation of the efficacy and safety of RW0117 on dyspepsia. https://cris.nih.go.kr/cris/search/detailSearch.do?seq=17281&search_... 2020;.:..
Kelber 2017 {published data only}
-
- Kelber O, Muller J, Vinson BR, Fink C, Abdel-Aziz H, Storr M, et al. The herbal medicine STW 5 is efficacious in targeting dyspeptic symptoms in all ages: A meta-analysis of randomized controlled trials. Neurogastroenterology and Motility 2017;29((Nieber) Institute fur Pharmacy, Leipzig, Germany):35.
Kim 2010 {published data only}
-
- Kim YM Park YC Jo JH Kang WC Son MW Hong KE. [Effect of Herb Medicine Treatment for Functional Dyspepsia: A Randomized Placebo-Controlled and Compared Standard Treatment Trial]. J Korean Oriental Med 2010;31(1):1.
Labenz 2023 {published data only}
-
- Labenz J, Anschütz M, Walstab J, Wedemeyer RS, Wolters H, Schug B (2023). Heartburn relief with bicarbonate-rich mineral water: results of the randomised, placebo-controlled phase-III trial STOMACH STILL. BMJ open gastroenterology 2023;10(1):e001048. [DOI: 10.1136/bmjgast-2022-001048] - DOI - PMC - PubMed
Lacy 2022 {published data only}
Lee 2020 {published data only}
-
- Lee DH, Shim HI, Shin CM, Yoon H, Park YS, Kim N, Seol SY. Inhibitory Effects of ß-caryophyllene on Helicobacter Pylori Infection: A Randomized Double-Blind, Placebo-Controlled Study. Digestive Endoscopy 2020;32(Supplement 1):196. - PubMed
Lopresti 2018 {published data only}
-
- ACTRN12619001514190. HerbagutA(R) for the treatment of digestive complaints in adults. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=378642 Date submitted: 29/10/2019.
Marakis 2002 {published data only}
-
- Bundy Rafe, Walker Ann F, Middleton Richard W, Marakis Georgios, Booth Jonathan C L. Artichoke leaf extract reduces symptoms of irritable bowel syndrome and improves quality of life in otherwise healthy volunteers suffering from concomitant dyspepsia: A subset analysis. Journal of Alternative and Complementary Medicine 2004;10(4):667-669. - PubMed
-
- Marakis G, Walker AF, Middleton RW, Booth JCL, Wright J, Pike DJ. Artichoke leaf extract reduces mild dyspepsia in an open study. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 2002;9(8):694-9. - PubMed
Meier 2005 {published data only}
-
- Meier R, Brignoli R. Artichoke leaf extract for functional dyspepsia. Result of an open, prospective, multicentre, phase IV trial. Swiss Journal of Integrative Medicine 2005;17(4):216-221.
Metugriachuk 2008 {published data only}
-
- Metugriachuk Y, Marotta F, Kuroi O, Tsuchiya J, Goh K-L, Minelli E, et al. Effect of a phyto-compound on delayed gastric emptying in functional dyspepsia: A randomized-controlled study. Journal of Digestive Diseases 2008;9(4):204-7. - PubMed
-
- Metugriachuk Y, Tsuchiya J, Marotta F, Kuroi O, Kawakita S, Pathak S, et al. Open-label randomized trial on the effectiveness of a herbal compound in selected patients affected by functional dyspepsia. International Medical Journal 2008;15(1):47-51.
Mihai 2019 {published data only}
-
- Mihai C, Mihai BM, Dranga M, Cardoneanu A, Prelipcean CC. Lactobacillus reuteri - An alternative in the first-line of helicobacter pylori eradication. Farmacia 2019;67(5):871-876.
Muss 2013 {published data only}
-
- Muss C, Mosgoeller W, Endler T. Papaya preparation (Caricol) in digestive disorders. Neuroendocrinology letters 2013;34(1):38-46. - PubMed
NCT04593836 {published data only}
-
- NCT04593836. Antiperistaltic Effect and Safety of L-menthol in the Elderly With Contraindication to Buscopan. https://clinicaltrials.gov/ct2/show/NCT04593836 First Posted: October 20, 2020.
Nili‐Ahmadabadi 2017 {published data only}
Pasalar 2015a {published data only}
-
- Pasalar M, Choopani R, Mosaddegh M, Kamalinejad M, Mohagheghzadeh A, Fattahi MR, et al. Efficacy of jollab in the treatment of depression in dyspeptic patients: a randomized double-blind controlled trial. Journal of evidence-based complementary & alternative medicine 2015;20(2):104-8. - PubMed
Shim 2015 {published data only}
-
- Shim YK, Kim N, Lee JY, Park YH, Yoon H, Shin CM, et al. The efficacy and safety of new prokinetkc agent benachio q soln. in patients with postprandial distress syndrome subtype in functional dyspepsia: A single-center, randomized, double-blind, placebocontrolled pilot study. United European Gastroenterology Journal 2015;3(5 SUPPL. 1):A648-9. - PubMed
-
- Shim YK, Lee JY, Kim NY, Park YH, Yoon H, Shin CM, et al. Efficacy and Safety of New Prokinetic Agent Benachio Q Solution in Patients with Postprandial Distress Syndrome Subtype in Functional Dyspepsia: A Single-center, Randomized, Double-blind, Placebo-controlled Pilot Study. Korean Journal Gastroenterology 2015;66(1):17-26. - PubMed
Shim 2019 {published data only}
-
- Shim HI Song DJ Shin CM Yoon H Park YS Kim N Lee DH. Inhibitory Effects of [beta]-caryophyllene on Helicobacter pylori Infection: a Randomized Double-blind, Placebo-controlled Study. The Korean Journal of Gastroenterology 2019;74(4):199-204. - PubMed
Wang 2017 {published data only}
-
- Wang Z, Chen F, Jiang W. Clinical study on amomum villosum lour extract in the treatment of functional dyspepsia due to deficiency of spleen and stomach. Biomedical Research 2017;28(21):9679-82.
Xiao 2013 {published data only}
-
- Xiao M, Qiu X, Yue D, Cai Y, Mo Q. Influence of hippophae rhamnoides on two appetite factors, gastric emptying and metabolic parameters, in children with functional dyspepsia. Hellenic Journal of Nuclear Medicine 2013;16(1):38-43. - PubMed
References to studies awaiting assessment
Borgia 1981 {published data only}
-
- Borgia M, Sepe N, Borgia R, Ori-Bellometti M. Pharmacological activity of a herbs extract: A controlled clinical study. Curr Ther Res 1981;29(3 II):525-36.
Borgia 1985 {published data only}
-
- Borgia M, Camarri E, Cataldi V, Frigerio G, Minerva V, Miracco A. [Double-blind double-controlled polycenter study on the therapeutic activity of a well-known combination of medicinal herbs]. La Clinica Terapeutica 1985;114(5):401-409. - PubMed
Chawla 1982 {published data only}
-
- Chawla Y K, Dubey P, Singh R, Nundy S, Tandon B N. Treatment of dyspepsia with Amalaki (Emblica officinalis Linn.)--an Ayurvedic drug. The Indian Journal of Medical Research 1982;76 Suppl(gjf, 0374701):95-98. - PubMed
CTRI/2018/02/012101 2018 {published data only}
-
- CTRI/2018/02/012101. To evaluate the effect of Pudin Hara Herbal Antacid Suspension in the management of Indigestion & Hyperacidity. http://www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=22909&... 2018.
Ernst 2002 {published data only}
-
- Ernst E. Red pepper alleviates functional dyspepsia. Focus on Alternative and Complementary Therapies 2002;7(3):245-246.
Fani 2007 {published data only}
-
- Fani A, Fani I, Delavar M, Fani P, Eshrati B, Elahi M. Combined garlic-omeprazole versus standard quadruple therapy for eradication of Helicobacter pylori infection. Indian Journal of Gastroenterology 2007;26(3):145-146. - PubMed
Fintelmann 1996 {published data only}
-
- Fintelmann V. Antidyspeptic and lipid lowering effects of artichoke (Cynara scolymus) extract. Results of clinical studies on efficacy and tolerability of Hepar-SL forte on 553 patients. Journal of General Medicine 1996;2(1):3-19.
Fintelmann 1999 {published data only}
-
- Fintelmann V. Artichoke extract in dyspeptic symptom complex. Methods and results of a clinical study. Zeitschrift fur Phytotherapie 1999;20(2):93-95.
Freise 1999 {published data only}
-
- Freise J, Kohler S. Peppermint oil/caraway oil-fixed combination in non-ulcer dyspepsia equivalent efficacy of the drug combination in an enteric coated or enteric soluble formulation. Die Pharmazie 1999;54(3):210-215. - PubMed
-
- Freise J, Kohler S. [Peppermint oil-caraway oil fixed combination in non-ulcer dyspepsia--comparison of the effects of enteric preparations]. Die Pharmazie 1999;54(3):210-215. - PubMed
IRCT20130211012438N27 {published data only}
-
- IRCT20130211012438N27. Evaluation of the effect of pyloricin soft gel on Helicobacter pylori infection. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20130211012438N27, 2018 2018.
IRCT20200128046291N1 {published data only}
-
- IRCT20200128046291N1. Evaluation of the effect of the traditional poly-herbal preparation, “Ghors-e-Vard”, on clinical symptoms of patients with functional dyspepsia. https://en.irct.ir/trial/45377 Registration date 2020-03-09.
ISRCTN31202330 {published data only}
-
- ISRCTN31202330. Aromatic plant intervention for gastrointestinal tract symptoms. http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN31202330, 2017 2017.
Ivashkin 2020 {published data only}
-
- Ivashkin V, Morozova M, Poluektova E, Shifrin O, Beniashvili A, Mamieva Z, Kovaleva A et al. In: United European Gastroenterology Journal. Vol. 8. 10-2020:565. [DOI: 10.1177/2050640620927345] - DOI
KCT0005020 {published data only}
-
- KCT0005020. A 8 week, Multi-center, Randomized, Double-blind, Placebo-Controlled Clinical Trial for the Evaluation of the Efficacy and Safety of CTE on Stomach Symptom. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02173164/... 2020.
Madisch 2000 {published data only}
-
- Madisch A Heydenrich CJ Wieland V Hufnagel R Hotz J. Equivalence of fixed Herbal combination preparation as compared with Cisapride in functional dyspepsia - influence on H. pylori - status (abstract). Gut 2000;47(Suppl 1):A111.
-
- Madisch A, Holtmann G, Schnitker J, Mayr G, Neuser J. Herbal preparation in functional dyspepsia - Hp-status is not a predictor for treatment success (abstract). N/A 2000;47(Suppl 1):A111.
Madisch 2001b {published data only}
-
- Madisch A, Holtmann G, Mayr G, Vinson B, Hotz J. Effectivity of the phytotherapeutic STW-5 II by functional dyspepsia - a randomised, placebo-controlled, double-blind study. Zeitschrift für Gastroenterologie 2002;40(8):703.
-
- Madisch A, Melderis H, Mayr G, Sassin I, Hotz J. A plant extract and its modified preparation in functional dyspepsia. Results of a double-blind placebo controlled comparative study. Zeitschrift für Gastroenterologie 2001;39(7):511-517. - PubMed
May 1996 {published data only}
-
- May B, Kuntz H-D, Kieser M, Kohler S. Efficacy of a fixed peppermint oil/caraway oil combination in non-ulcer dyspepsia. Arzneimittelforschung 1996;46(12):1149-1153. - PubMed
NCT04742985 {published data only}
-
- NCT04742985. Effects of Combined Extracts of Green Tea Seed (Saponins) and Green Tea Leaves (Epigallocatechin-3-gallate) on Gastric Mucosal Protection: A Pilot Study. https://clinicaltrials.gov/ct2/show/NCT04742985 First Posted: February 8, 2021.
Rosch 2001 {published data only}
-
- Rosch W Vinson B. Phytotherapy by functional dyspepsia. Cisaprid versus Iberogast in dysmotility dyspepsia. Zeitschrift für Gastroenterologie 2001;39(10):893.
Roth 1996 {published data only}
-
- Roth R. Phytotherapy in functional gastrointestinal diseases. Successful phytopharmacotherapy of irritable stomach and colon. Therapiewoche 1996;46(10):549-52.
TCTR20180530003 {published data only}
-
- TCTR20180530003. The efficacy of curcuminoid in patients with overlapping gastroesophageal reflux disease and functional dyspepsia: a randomized controlled trial. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01907301/... 2018.
References to ongoing studies
CTRI/2020/04/024448 {published data only}
-
- CTRI/2020/04/024448. A clinical study to check the efficacy and safety of investigational product in indigestion. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=41893&EncHid... Registered on: 03/04/2020.
IRCT20090527001957N8 {published data only}
-
- IRCT20090527001957N8. Effect of Pistacia lentiscus in functional dyspepsia. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20090527001957N8 2019.
IRCT20150927024228N2 {published data only}
-
- IRCT20150927024228N2. Efficacy of Apium graveolense and Trachyspermum copticum on postprandial syndrome. https://en.irct.ir/trial/37515?revision=82438 2019.
IRCT20160721029026N4 {published data only}
-
- IRCT20160721029026N4. Herbal Drug for Treatment of Functional Dyspepsia and eradication of Helicobacter pylori(H.pylori) infection. https://en.irct.ir/trial/23418 2017.
IRCT20170802035460N2 {published data only}
-
- IRCT20170802035460N2. Effect of Cinnamon Oil Soft Capsule on Functional Dyspepsia. https://en.irct.ir/trial/27767 2017.
IRCT20190304042911N1 {published data only}
-
- IRCT20190304042911N1. Evaluating Efficacy of traditional preparation containing Bunium persicum and Coriandrum sativum on clinical symptoms of patients with functional dyspepsia. https://en.irct.ir/trial/38380 2019.
IRCT20190806044456N1 {published data only}
-
- IRCT20190806044456N1. Comparison of the effects of herbal capsule Govarcin and Metoclopramide on Treatment of functional dyspepsia. https://en.irct.ir/trial/41369 2019.
IRCT20200303046677N6 {published data only}
-
- IRCT20200303046677N6. The effect of compound honey syrup on Functional Dyspepsia: a randomized double-blind placebo-controlled clinical trial. https://en.irct.ir/trial/52650 Registration date: 14-02-2021.
IRCT20200424047192N1 {published data only}
-
- IRCT20200424047192N1. Comparative study of the effect of eating and drinking modification and sumac capsule with omeprazole on functional dyspepsia symptoms in adults. https://en.irct.ir/trial/47931 Registration date: 24-01-2021.
IRCT20220304054179N1 {published data only}
-
- IRCT20220304054179N1. The efficacy of an Iranian traditional herbal compound in treatment of dyspepsia. en.irct.ir/trial/62308 Date of registration: 13 April 2022.
KCT0005229 {published data only}
-
- KCT0005229. A 12 week, Randomized, Double-blind, Placebo-Controlled Clinical Trial for the Evaluation of the Efficacy and Safety of EDL on Dyspepsia. https://cris.nih.go.kr/cris/search/detailSearch.do?seq=17197&search_... 2020. - PMC - PubMed
NCT03548363 {published data only}
-
- NCT03548363. Effect of Gingest on Symptoms of Dyspepsia. https://clinicaltrials.gov/ct2/show/NCT03548363 2018.
NCT04482478 {published data only}
-
- NCT04482478. Clinical Trial for the Evaluation of the Efficacy and Safety of EDL on Dyspepsia. https://clinicaltrials.gov/ct2/show/NCT04482478 First Posted: July 22, 2020.
NCT04656730 {published data only}
-
- EUCTR2019-003976-38-ES. Study to asess the efects of IberogastA(R) and IberogastA(R) N in intestinal gas transit and abdominal symptoms of patients suffering from irritable bowel syndrom or functional dyspsia. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-003976-38-ES, 2020 2020.
-
- NCT04656730. Effect of STW5 (Iberogast (R)) and STW5-II (Iberogast N(R)) on Transit and Tolerance of Intestinal Gas. https://clinicaltrials.gov/ct2/show/NCT04656730 First Posted: December 7, 2020.
RBR‐10yqwrk6 {published data only}
-
- RBR-10yqwrk6. Double-blind randomized clinical trial of Maytenus ilicifolia as a strategy to manage dyspepsia in gastroesophageal reflux disease. https://ensaiosclinicos.gov.br/rg/RBR-10yqwrk6 Date of registration: 03/26/2021.
Additional references
Aziz 2018
-
- Aziz I, Palsson OS, Törnblom H, Sperber AD, Whitehead WE, Simrén M. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: a cross-sectional population-based study [Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: a cross-sectional population-based study]. Lancet Gastroenterol Hepatol January 29, 2018;3(4):252-262. - PubMed
Bang 2015
-
- Bang CS, Kim JH, Baik GH, Kim HS, Park SH, Kim EJ, et al. Mosapride treatment for functional dyspepsia: a meta-analysis. Journal of Gastroenterology and Hepatology 2015;30(1):28-42. - PubMed
Bent 2008
Bodagh 2018
Boon 2021
Brun 2010
Carbone 2017
Chassany 2012
-
- Chassany O, Shaheen NJ, Karlsson M, Hughes N, Ryden A. Systematic review: symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scandinavian Journal of Gastroenterology 2012;47(12):1412-21. [PMID: ] - PubMed
Chiarioni 2018
Choung 2012
Ekor 2014
Ford 2010
-
- Ford AC, Marwaha A, Lim A, Moayyedi P. Systematic review and meta-analysis of the prevalence of irritable bowel syndrome in individuals with dyspepsia. Clinical Gastroenterology and Hepatology 2010;8(5):401-9. [PMID: ] - PubMed
Ford 2020
-
- Ford, AC, Moayyedi, P, Black, CJ et al. Systematic review and network meta-analysis: efficacy of drugs for functional dyspepsia. Alimentary pharmacology & therapeutics 16 September 2020;53(1):8-21. - PubMed
GRADEpro GDT 2020 [Computer program]
-
- GRADEpro GDT: GRADEpro Guideline Development Tool. Version accessed 11 August 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Higgins 2003
Higgins 2008
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from www.handbook.cochrane.org.. The Cochrane Collaboration, 2011.
Higgins 2022
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Available from www.training.cochrane.org/handbook.. Cochrane, 2022.
Holtmann 2004
-
- Holtmann G, Adam B, Vinson B. Evidence-based medicine and phytotherapy for functional dyspepsia and irritable bowel syndrome: A systematic analysis of evidence for the herbal preparation Iberogast. Wiener Medizinische Wochenschrift 2004;154(21-22):528-534. - PubMed
Huang 2012
Hungin 2009
-
- Hungin AP, Hill C, Raghunath A. Systematic review: frequency and reasons for consultation for gastro-oesophageal reflux disease and dyspepsia. Alimentary Pharmacology & Therapeutics 2009;30(4):331-42. - PubMed
Komori 2019
Krueger 2004
-
- Krueger KJ, Dryden GW, McClain CJ. Complementary and alternative medicine therapies for gastrointestinal disease. Gastroenterology 2004;10(Part 4):1.
Lacy 2012
Lan 2014
Langmead 2001
-
- Langmead L, Rampton DS. Review article: herbal treatment in gastrointestinal and liver disease—benefits and dangers. Alimentary Pharmacology & Therapeutics 2001;15(9):1239-52. - PubMed
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, Noel-Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Chapter 4: Searching for and selecting studies.. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated February 2021). Available from www.training.cochrane.org/handbook. Cochrane, 2021.
Mahadeva 2006
McKenzie 2021
-
- McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Available from www.training.cochrane.org/handbook.. Cochrane, 2021.
Meier 2013
-
- Meier R, Hengstler P, Weber F, Maurer H, Bommeli C, Brignoli R. The Tibetan herbal formula Padma Digestin in functional dyspepsia: an open-label study. Forschende Komplementarmedizin (2006) 2013;20(Suppl 2):2-7. - PubMed
Melzer 2004
-
- Melzer J, Rosch W, Reichling J, Brignoli R, Saller R. Meta-analysis: phytotherapy of functional dyspepsia with the herbal drug preparation STW 5 (Iberogast). Alimentary Pharmacology & Therapeutics 2004;20(11-12):1279-87. - PubMed
Melzer J 2004
-
- Melzer J, Iten F, Reichling J, Saller R. Iberis amara L. and Iberogast--results of a systematic review concerning functional dyspepsia. Journal of Herbal Pharmacotherapy 2004;4(4):51-59. - PubMed
Moayyedi 2004
-
- Moayyedi P, Delaney BC, Vakil N, Forman D, Talley NJ. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology 2004;127(5):1329-37. - PubMed
Moayyedi 2006
Montoro 2004
-
- Montoro Huguet MA, Santolaria Piedrafita S. Quality of life in patients with functional dyspepsia [Calidad de vida en los pacientes con dispepsia funcional]. Gastroenterologia y Hepatologia 2004;27:15-23.
NIH 2016
-
- National Center for Complementary and Integrative Health. Complementary, alternative, or integrative health: what’s in a name? nccih.nih.gov/health/integrative-health (accessed 1 September 2017).
Page 2021
Park 2010
Pesce 2020
Pinto 2017
Pittayanon 2018
RevMan Web 2021 [Computer program]
-
- Review Manager Web (RevMan Web). Version 3.6.0. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Saller 2002
-
- Saller R, Pfister-Hotz G, Iten F, Melzer J, Reichling J. Iberogast: A modern phytotherapeutic combined herbal drug for the treatment of functional disorders of the gastrointestinal tract (dyspepsia, irritable bowel syndrome) - From phytomedicine to 'evidence based phytotherapy'. A systematic review. Forschende Komplementarmedizin und Klassische Naturheilkunde 2002;9(SUPPL. 1):1-20. - PubMed
Schünemann 2021
-
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, Akl EA, Guyatt GH. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Available from www.training.cochrane.org/handbook.. Cochrane, 2021.
Shaw 2012
-
- Shaw D, Graeme L, Pierre D, Elizabeth W, Kelvin C. Pharmacovigilance of herbal medicine. Journal of Ethnopharmacology 2012;140(3):513-8. - PubMed
Shim 2015
-
- Shim YK, Lee JY, Kim NY, Park YH, Yoon H, Shin CM, et al. Efficacy and safety of new prokinetic agent Benachio Q Solution(r) in patients with postprandial distress syndrome subtype in functional dyspepsia: a single-center, randomized, double-blind, placebo-controlled pilot study. Taehan Sohwagi Hakhoe Chi [Korean Journal of Gastroenterology] 2015;66(1):17-26. - PubMed
Stanghellini 2014
-
- Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut 2014;63(12):1972-8. - PubMed
Stanghellini 2016
-
- Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal disorders. Gastroenterology 2016;150(6):1380-92. - PubMed
Tack 2004
Tack 2006
-
- Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology 2006;130(5):1466-79. - PubMed
Talley 1991
-
- Talley NJ, Colin-Jones D, Koch KL, et al. Functional dyspepsia. A classification with guidelines for diagnosis and management.. Gastroenterol International 1991;4:145-60.
Talley 1999
Talley 2015
-
- Talley NJ, Ford AC. Functional dyspepsia. New England Journal of Medicine 2015;373(19):1853-63. - PubMed
Talley 2017
Teschke 2015
Thompson 2002
-
- Thompson Coon J, Ernst E. Systematic review: herbal medicinal products for non-ulcer dyspepsia. Alimentary Pharmacology & Therapeutics 2002;16(10):1689-99. - PubMed
Thomson 2013
Tilburt 2008
Tillisch 2006
Vanheel 2017
Vlachojannis 2010
-
- Vlachojannis J, Cameron M, Chrubasik S. Medicinal use of potato-derived products: A systematic review. Phytotherapy Research 2010;24(2):159-162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

