Cystic fibrosis modulator therapy can reverse cystic bronchiectasis
- PMID: 37323158
- PMCID: PMC10261305
- DOI: 10.1002/rcr2.1172
Cystic fibrosis modulator therapy can reverse cystic bronchiectasis
Abstract
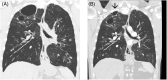
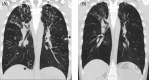
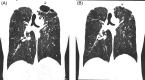
Bronchiectasis is often considered progressive and irreversible, so cases of regression or reversal are an important step in understanding the underlying pathophysiological mechanisms. Cystic fibrosis, (CF) caused by pathogenic variants in the cystic fibrosis transmembrane conductance regulator (CFTR) gene has been a success story in personalized medicine. The recent development of CFTR modulator therapies has revolutionized care. Dramatic improvements in lung function, sputum production, daytime functioning, and quality of life are seen within weeks. However, the effect of long-term exposure to elexacaftor + tezacaftor + ivacaftor (ETI) on the structural abnormalities is at present unknown. This case series outlines three adults with CF who have demonstrated progressive improvement in the cylindrical, varicose and importantly cystic changes of bronchiectasis with prolonged ETI treatment. This raises the exciting question of reversibility of bronchiectasis as well as the mechanisms involved in the maintenance and progression of bronchiectasis as it relates to CF.
Keywords: bronchiectasis; cystic fibrosis; elexacaftor; ivacaftor; tezacaftor.
© 2023 The Authors. Respirology Case Reports published by John Wiley & Sons Australia, Ltd on behalf of The Asian Pacific Society of Respirology.
Conflict of interest statement
Peter Middleton reports grants from Vertex Pharmaceuticals, during the conduct of the study; personal fees from Vertex Pharmaceuticals, outside the submitted work. Nicholas Simmonds reports personal fees from Vertex Pharmaceuticals, Chiesi, Gilead, Menarini, Zambon, outside the submitted work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources