Biomaterials and nanomedicine for bone regeneration: Progress and future prospects
- PMID: 37323213
- PMCID: PMC10190996
- DOI: 10.1002/EXP.20210011
Biomaterials and nanomedicine for bone regeneration: Progress and future prospects
Abstract
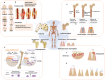
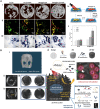
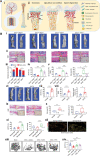
Bone defects pose a heavy burden on patients, orthopedic surgeons, and public health resources. Various pathological conditions cause bone defects including trauma, tumors, inflammation, osteoporosis, and so forth. Auto- and allograft transplantation have been developed as the most commonly used clinic treatment methods, among which autologous bone grafts are the golden standard. Yet the repair of bone defects, especially large-volume defects in the geriatric population or those complicated with systemic disease, is still a challenge for regenerative medicine from the clinical perspective. The fast development of biomaterials and nanomedicine favors the emergence and promotion of efficient bone regeneration therapies. In this review, we briefly summarize the progress of novel biomaterial and nanomedical approaches to bone regeneration and then discuss the current challenges that still hinder their clinical applications in treating bone defects.
Keywords: biomaterials; bone defects; nanomedicine; scaffolds; tissue engineering.
© 2021 The Authors. Exploration published by Henan University and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Wei Tao and Omid C. Farokhzad are members of the Exploration editorial board. Omid C. Farokhzad has financial interests in Selecta Biosciences, Tarveda Therapeutics, XLINK Therapeutics, PrognomIQ, and Seer.
Figures


Similar articles
-
Applications of X-ray computed tomography for the evaluation of biomaterial-mediated bone regeneration in critical-sized defects.J Microsc. 2020 Mar;277(3):179-196. doi: 10.1111/jmi.12844. Epub 2019 Nov 20. J Microsc. 2020. PMID: 31701530 Review.
-
Ion incorporation into bone grafting materials.Periodontol 2000. 2024 Feb;94(1):213-230. doi: 10.1111/prd.12533. Epub 2023 Oct 12. Periodontol 2000. 2024. PMID: 37823468 Review.
-
Stem Cell-Friendly Scaffold Biomaterials: Applications for Bone Tissue Engineering and Regenerative Medicine.Front Bioeng Biotechnol. 2020 Dec 14;8:598607. doi: 10.3389/fbioe.2020.598607. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33381499 Free PMC article. Review.
-
Delivering Nucleic-Acid Based Nanomedicines on Biomaterial Scaffolds for Orthopedic Tissue Repair: Challenges, Progress and Future Perspectives.Adv Mater. 2016 Jul;28(27):5447-69. doi: 10.1002/adma.201505088. Epub 2016 Feb 3. Adv Mater. 2016. PMID: 26840618 Review.
-
Biomaterial scaffolds regulate macrophage activity to accelerate bone regeneration.Front Bioeng Biotechnol. 2023 Feb 2;11:1140393. doi: 10.3389/fbioe.2023.1140393. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36815893 Free PMC article. Review.
Cited by
-
Novel Approaches and Biomaterials for Bone Tissue Engineering: A Focus on Silk Fibroin.Materials (Basel). 2022 Oct 7;15(19):6952. doi: 10.3390/ma15196952. Materials (Basel). 2022. PMID: 36234293 Free PMC article. Review.
-
Electrospun naringin-loaded microsphere/sucrose acetate isobutyrate system promotes macrophage polarization toward M2 and facilitates osteoporotic bone defect repair.Regen Biomater. 2023 Feb 20;10:rbad006. doi: 10.1093/rb/rbad006. eCollection 2023. Regen Biomater. 2023. PMID: 36911145 Free PMC article.
-
Intelligent Vascularized 3D/4D/5D/6D-Printed Tissue Scaffolds.Nanomicro Lett. 2023 Oct 31;15(1):239. doi: 10.1007/s40820-023-01187-2. Nanomicro Lett. 2023. PMID: 37907770 Free PMC article. Review.
-
Elimination of methicillin-resistant Staphylococcus aureus biofilms on titanium implants via photothermally-triggered nitric oxide and immunotherapy for enhanced osseointegration.Mil Med Res. 2023 May 4;10(1):21. doi: 10.1186/s40779-023-00454-y. Mil Med Res. 2023. PMID: 37143145 Free PMC article.
-
Extracellular vesicles as bioactive nanotherapeutics: An emerging paradigm for regenerative medicine.Theranostics. 2022 Jun 21;12(11):4879-4903. doi: 10.7150/thno.72812. eCollection 2022. Theranostics. 2022. PMID: 35836815 Free PMC article.
References
-
- Zhang X., Koo S., Kim J. H., Huang X., Kong N., Zhang L., Zhou J., Xue J., Harris M. B., Tao W., Kim J. S., Matter 2021, 4, 2727.
LinkOut - more resources
Full Text Sources
