Lead-free double perovskite Cs2MBiCl6 (M = Ag, Cu): insights into the optical, dielectric, and charge transfer properties
- PMID: 37323437
- PMCID: PMC10261914
- DOI: 10.1039/d3ra02731g
Lead-free double perovskite Cs2MBiCl6 (M = Ag, Cu): insights into the optical, dielectric, and charge transfer properties
Abstract
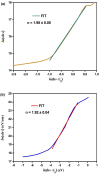
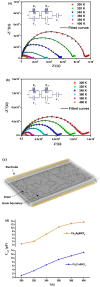
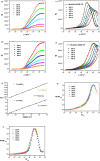
Recently, double perovskites have shown excellent potential considering the instability and toxicity problems of lead halide perovskites in optoelectronic devices. Here, the double perovskites Cs2MBiCl6 (M = Ag, Cu) were successfully synthesized via the slow evaporation solution growth technique. The cubic phase of these double perovskite materials was verified through the X-ray diffraction pattern. The investigation of Cs2CuBiCl6 and Cs2AgBiCl6 utilizing optical analysis showed that their respective indirect band-gap values were 1.31 and 2.92 eV, respectively. These materials, which are double perovskites, were examined using the impedance spectroscopy technique within the 10-1 to 106 Hz frequency and 300-400 K temperature ranges. Jonncher's power law was utilized to describe AC conductivity. The outcomes of the study on charge transportation in Cs2MBiCl6 (where M = Ag, Cu) suggest that the non-overlapping small polaron tunneling mechanism was present in Cs2CuBiCl6, whereas the overlapping large polaron tunneling mechanism was present in Cs2AgBiCl6.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








References
-
- Akman E. Ozturk T. Xiang W. Sadegh F. Prochowicz D. Tavakoli M. M. Yadav P. Yilmaz M. Akin S. Energy Environ. Sci. 2023;16(2):372.
-
- Öztürk T. Akman E. Shalan A. E. Akin S. Nano Energy. 2021;87:106157.
-
- Xiang W. Wang Z. Kubicki D. J. Tress W. Luo J. Prochowicz D. Akin S. Emsley L. Zhou J. Dietler G. Grätzel M. Hagfeldt A. Joule. 2019;3(1):205.
-
- Luo X. Lai R. Li Y. Han Y. Liang G. Liu X. Ding T. Wang J. Wu K. J. Am. Chem. Soc. 2019;141:4186. - PubMed
-
- Pradeep K. R. Viswanatha R. Mater. Res. Bull. 2021;141:5.
LinkOut - more resources
Full Text Sources
Miscellaneous

