Change in Methicillin-Resistant Staphylococcus aureus Testing in the Intensive Care Unit as an Antimicrobial Stewardship Initiative
- PMID: 37323512
- PMCID: PMC10262952
- DOI: 10.31486/toj.22.0103
Change in Methicillin-Resistant Staphylococcus aureus Testing in the Intensive Care Unit as an Antimicrobial Stewardship Initiative
Abstract
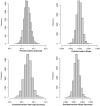
Background: Methicillin-resistant Staphylococcus aureus (MRSA)-associated infections are a cause of morbidity and mortality in the intensive care unit (ICU). Vancomycin is a treatment option but is not without risks. Methods: A MRSA testing change-the switch from culture to polymerase chain reaction-was implemented at 2 adult (tertiary and community) ICUs located in a Midwestern US health system. Data from 2016 to 2020 were included in the study, and the median change in time to test results was examined. Results: During the study period, 71% of 19,975 patients seen at the 2 ICUs received MRSA testing. In the preintervention period, 91% and 99% of patients at the tertiary and community hospitals received testing via culture, respectively. Culture testing was used 1% and ∼0% of the time at the tertiary and community hospitals, respectively, in the postintervention period. A counterfactual estimate showed 36 (95% credible interval [CrI], 35, 37) and 32 (95% CrI, 31, 33) fewer hours until results were available at the tertiary and community hospitals, respectively. Conclusion: After the testing change, MRSA results were available in less time. Obtaining results sooner can assist with antimicrobial stewardship through the potential delay in initiating therapies such as vancomycin and/or quicker de-escalation of such therapies.
Keywords: Anti-infective agents; Staphylococcus aureus; diagnosis; diagnostic techniques and procedures; intensive care units; methicillin-resistant Staphylococcus aureus; vancomycin.
©2023 by the author(s); Creative Commons Attribution License (CC BY).
Figures




Similar articles
-
Methicillin-resistant Staphylococcus aureus nasal colonization is a poor predictor of intensive care unit-acquired methicillin-resistant Staphylococcus aureus infections requiring antibiotic treatment.Crit Care Med. 2010 Oct;38(10):1991-5. doi: 10.1097/CCM.0b013e3181eeda3f. Crit Care Med. 2010. PMID: 20683260
-
Methicillin-resistant Staphylococcus aureus in German intensive care units during 2000-2003: data from Project SARI (Surveillance of Antimicrobial Use and Antimicrobial Resistance in Intensive Care Units).Infect Control Hosp Epidemiol. 2006 Feb;27(2):146-54. doi: 10.1086/500619. Epub 2006 Feb 8. Infect Control Hosp Epidemiol. 2006. PMID: 16465631
-
Active surveillance testing and decontamination strategies in intensive care units to reduce methicillin-resistant Staphylococcus aureus infections.Am J Infect Control. 2010 Jun;38(5):361-7. doi: 10.1016/j.ajic.2009.09.018. Epub 2010 Feb 26. Am J Infect Control. 2010. PMID: 20189267
-
Methicillin-resistant Staphylococcus aureus in Intensive Care Unit Setting of India: A Review of Clinical Burden, Patterns of Prevalence, Preventive Measures, and Future Strategies.Indian J Crit Care Med. 2020 Jan;24(1):55-62. doi: 10.5005/jp-journals-10071-23337. Indian J Crit Care Med. 2020. PMID: 32148350 Free PMC article. Review.
-
European perspective and update on the management of nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid.Clin Microbiol Infect. 2014 Apr;20 Suppl 4:19-36. doi: 10.1111/1469-0691.12450. Clin Microbiol Infect. 2014. PMID: 24580739 Review.
References
LinkOut - more resources
Full Text Sources
