Carrier strategies boost the application of CRISPR/Cas system in gene therapy
- PMID: 37323878
- PMCID: PMC10190933
- DOI: 10.1002/EXP.20210081
Carrier strategies boost the application of CRISPR/Cas system in gene therapy
Abstract
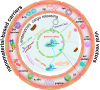
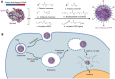
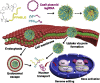
Emerging clustered regularly interspaced short palindromic repeat/associated protein (CRISPR/Cas) genome editing technology shows great potential in gene therapy. However, proteins and nucleic acids suffer from enzymatic degradation in the physiological environment and low permeability into cells. Exploiting carriers to protect the CRISPR system from degradation, enhance its targeting of specific tissues and cells, and reduce its immunogenicity is essential to stimulate its clinical applications. Here, the authors review the state-of-the-art CRISPR delivery systems and their applications, and describe strategies to improve the safety and efficacy of CRISPR mediated genome editing, categorized by three types of cargo formats, that is, Cas: single-guide RNA ribonucleoprotein, Cas mRNA and single-guide RNA, and Cas plasmid expressing CRISPR/Cas systems. The authors hope this review will help develop safe and efficient nanomaterial-based carriers for CRISPR tools.
Keywords: CRISPR; drug delivery; gene therapy; genome editing; nanomaterials.
© 2022 The Authors. Exploration published by Henan University and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




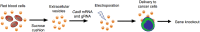

Similar articles
-
Cell-derived extracellular vesicles for CRISPR/Cas9 delivery: engineering strategies for cargo packaging and loading.Biomater Sci. 2022 Jul 26;10(15):4095-4106. doi: 10.1039/d2bm00480a. Biomater Sci. 2022. PMID: 35766814 Review.
-
Rational designs of in vivo CRISPR-Cas delivery systems.Adv Drug Deliv Rev. 2021 Jan;168:3-29. doi: 10.1016/j.addr.2019.11.005. Epub 2019 Nov 21. Adv Drug Deliv Rev. 2021. PMID: 31759123 Review.
-
[Genome editing in plants directed by CRISPR/Cas ribonucleoprotein complexes].Yi Chuan. 2020 Jun 20;42(6):556-564. doi: 10.16288/j.yczz.20-017. Yi Chuan. 2020. PMID: 32694114 Review. Chinese.
-
Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing.Biologics. 2021 Aug 21;15:353-361. doi: 10.2147/BTT.S326422. eCollection 2021. Biologics. 2021. PMID: 34456559 Free PMC article. Review.
-
Efficient expression of multiple guide RNAs for CRISPR/Cas genome editing.aBIOTECH. 2020 Jan 23;1(2):123-134. doi: 10.1007/s42994-019-00014-w. eCollection 2020 Apr. aBIOTECH. 2020. PMID: 36304720 Free PMC article. Review.
Cited by
-
Thermus thermophilus Argonaute-based signal amplifier for highly sensitive and specific microRNA detection.Front Bioeng Biotechnol. 2023 Jul 31;11:1221943. doi: 10.3389/fbioe.2023.1221943. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37583711 Free PMC article.
-
Exploring Advanced CRISPR Delivery Technologies for Therapeutic Genome Editing.Small Sci. 2024 Jul 25;4(10):2400192. doi: 10.1002/smsc.202400192. eCollection 2024 Oct. Small Sci. 2024. PMID: 40212235 Free PMC article.
-
CRISPR/Cas-mediated "one to more" lighting-up nucleic acid detection using aggregation-induced emission luminogens.Nat Commun. 2024 Oct 3;15(1):8560. doi: 10.1038/s41467-024-52931-0. Nat Commun. 2024. PMID: 39362874 Free PMC article.
-
Erythrocyte-Leveraged Oncolytic Virotherapy (ELeOVt): Oncolytic Virus Assembly on Erythrocyte Surface to Combat Pulmonary Metastasis and Alleviate Side Effects.Adv Sci (Weinh). 2024 Feb;11(5):e2303907. doi: 10.1002/advs.202303907. Epub 2023 Nov 23. Adv Sci (Weinh). 2024. PMID: 37997186 Free PMC article.
-
The Combination of Lactoferrin and Creatine Ameliorates Muscle Decay in a Sarcopenia Murine Model.Nutrients. 2024 Jun 19;16(12):1958. doi: 10.3390/nu16121958. Nutrients. 2024. PMID: 38931310 Free PMC article.
References
-
- (a) Dunbar C. E., High K. A., Joung J. K., Kohn D. B., Ozawa K., Sadelain M., Science 2018, 359, eaan4672; - PubMed
- (b) Huo S., Gong N., Jiang Y., Chen F., Guo H., Gan Y., Wang Z., Herrmann A., Liang X.‐J., Sci. Adv. 2019, 5, eaaw6264; - PMC - PubMed
- (c) Kulkarni J. A., Witzigmann D., Thomson S. B., Chen S., Leavitt B. R., Cullis P. R., van der Meel R., Nat. Nanotechnol. 2021, 16, 630. - PubMed
-
- (a) Wittrup A., Lieberman J., Nat. Rev. Genet. 2015, 16, 543; - PMC - PubMed
- (b) Sahin U., Kariko K., Tuereci O., Nat. Rev. Drug. Discovery 2014, 13, 759; - PubMed
- (c) Guo S., Huang Y., Jiang Q., Sun Y., Deng L., Liang Z., Du Q., Xing J., Zhao Y., Wang P. C., Dong A., Liang X.‐J., ACS Nano 2010, 4, 5505; - PMC - PubMed
- (d) Zhang T., Huang Y., Ma X., Gong N., Liu X., Liu L., Ye X., Hu B., Li C., Tian J.‐H., Magrini A., Zhang J., Guo W., Xing J.‐F., Bottini M., Liang X.‐J., Nano Lett. 2018, 18, 6301; - PubMed
- (e) Weng Y., Xiao H., Zhang J., Liang X.‐J., Huang Y., Biotechnol. Adv. 2019, 37, 801. - PubMed
-
- Wei C., Liu J., Yu Z., Zhang B., Gao G., Jiao R., J. Genet. Genomics 2013, 40, 281. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
