Rational design of allosteric switchable catalysts
- PMID: 37323883
- PMCID: PMC10191014
- DOI: 10.1002/EXP.20210095
Rational design of allosteric switchable catalysts
Abstract
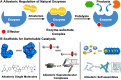
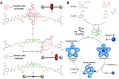
Allosteric regulation, in many cases, involves switching the activities of natural enzymes, which further affects the enzymatic network and cell signaling in the living systems. The research on the construction of allosteric switchable catalysts has attracted broad interests, aiming to control the progress and asymmetry of catalytic reactions, expand the chemical biology toolbox, substitute unstable natural enzymes in the biological detection and biosensors, and fabricate the biomimetic cascade reactions. Thus, in this review, we summarize the recent outstanding works in switchable catalysts based on the allosterism of single molecules, supramolecular complexes, and self-assemblies. The concept of allosterism was extended from natural proteins to polymers, organic molecules, and supramolecular systems. In terms of the difference between these building scaffolds, a variety of design methods that tailor biological and synthetic molecules into controllable catalysts were introduced with emphasis.
Keywords: allosterism; molecular machine; self‐assembly; supramolecular catalysis; switchable catalyst.
© 2022 The Authors. Exploration published by Henan University and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Xue Xue is a member of the Exploration editorial board. The authors declare no conflict of interest.
Figures







References
-
- Pratsinis A., Kelesidis G. A., Zuercher S., Krumeich F., Bolisetty S., Mezzenga R., Leroux J. C., Sotiriou G. A., ACS Nano 2017, 11, 12210. - PubMed
-
- Kwok R. T. K., Leung C. W. T., Lam J. W. Y., Tang B. Z., Chem. Soc. Rev. 2015, 44, 4228. - PubMed
-
- Liang W., Wied P., Carraro F., Sumby C. J., Nidetzky B., Tsung C. K., Falcaro P., Doonan C. J., Chem. Rev. 2021, 121, 1077. - PubMed
-
- Jemli S., Ayadi‐Zouari D., Ben Hlima H., Bejar S., Crit. Rev. Biotechnol. 2014, 8551, 1–13. - PubMed
-
- Jun L. Y., Yon L. S., Mubarak N. M., Bing C. H., Pan S., Danquah M. K., Abdullah E. C., Khalid M., J. Environ. Chem. Eng. 2019, 7, 102961.
Publication types
LinkOut - more resources
Full Text Sources
