Electrolyte formulation strategies for potassium-based batteries
- PMID: 37323885
- PMCID: PMC10191034
- DOI: 10.1002/EXP.20210239
Electrolyte formulation strategies for potassium-based batteries
Abstract
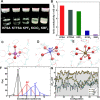
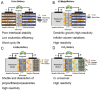
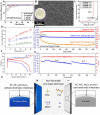
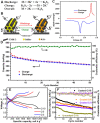
Potassium (K)-based batteries are viewed as the most promising alternatives to lithium-based batteries, owing to their abundant potassium resource, lower redox potentials (-2.97 V vs. SHE), and low cost. Recently, significant achievements on electrode materials have boosted the development of potassium-based batteries. However, the poor interfacial compatibility between electrode and electrolyte hinders their practical. Hence, rational design of electrolyte/electrode interface by electrolytes is the key to develop K-based batteries. In this review, the principles for formulating organic electrolytes are comprehensively summarized. Then, recent progress of various liquid organic and solid-state K+ electrolytes for potassium-ion batteries and beyond are discussed. Finally, we offer the current challenges that need to be addressed for advanced K-based batteries.
Keywords: fundamentals of organic electrolytes; liquid electrolytes; potassium‐based batteries; solid‐state electrolytes.
© 2022 The Authors. Exploration published by Henan University and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
There are no conflicts to declare.
Figures














References
-
- Zeng X., Li M., El‐Hady D. A., Alshitari W., Al‐Bogami A. S., Lu J., Amine K., Adv. Energy Mater. 2019, 9, 1900161.
-
- a) Liu P., Wang Y., Hao H., Basu S., Feng X., Xu Y., Boscoboinik J. A., Nanda J., Watt J., Mitlin D., Adv. Mater. 2020, 32, 2002908; - PubMed
- b) Li J., Gao W., Huang L., Jiang Y., Chang X., Sun S., Pan L., Appl. Surf. Sci. 2022. 571, 151307.
-
- Matsuura N., Umemoto K., Takeuchi Z. i., Bull. Chem. Soc. Jpn. 1974, 47, 813.
-
- Matsuda Y., Nakashima H., Morita M., Takasu Y., J. Electrochem. Soc. 1981, 128, 2552.
Publication types
LinkOut - more resources
Full Text Sources
