Physiological changes during torpor favor association with Endozoicomonas endosymbionts in the urochordate Botrylloides leachii
- PMID: 37323901
- PMCID: PMC10264598
- DOI: 10.3389/fmicb.2023.1072053
Physiological changes during torpor favor association with Endozoicomonas endosymbionts in the urochordate Botrylloides leachii
Abstract
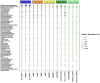
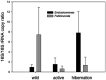
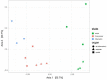
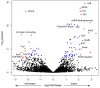
Environmental perturbations evoke down-regulation of metabolism in some multicellular organisms, leading to dormancy, or torpor. Colonies of the urochordate Botrylloides leachii enter torpor in response to changes in seawater temperature and may survive for months as small vasculature remnants that lack feeding and reproductive organs but possess torpor-specific microbiota. Upon returning to milder conditions, the colonies rapidly restore their original morphology, cytology and functionality while harboring re-occurring microbiota, a phenomenon that has not been described in detail to date. Here we investigated the stability of B. leachii microbiome and its functionality in active and dormant colonies, using microscopy, qPCR, in situ hybridization, genomics and transcriptomics. A novel lineage of Endozoicomonas, proposed here as Candidatus Endozoicomonas endoleachii, was dominant in torpor animals (53-79% read abundance), and potentially occupied specific hemocytes found only in torpid animals. Functional analysis of the metagenome-assembled genome and genome-targeted transcriptomics revealed that Endozoicomonas can use various cellular substrates, like amino acids and sugars, potentially producing biotin and thiamine, but also expressing various features involved in autocatalytic symbiosis. Our study suggests that the microbiome can be linked to the metabolic and physiological states of the host, B. leachii, introducing a model organism for the study of symbioses during drastic physiological changes, such as torpor.
Keywords: Endozoicomonas; aestivation; ascidians; hibernation; metabolism; symbiosis; torpor.
Copyright © 2023 Hyams, Rubin-Blum, Rosner, Brodsky, Rinkevich and Rinkevich.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Insights into the unique torpor of Botrylloides leachi, a colonial urochordate.Dev Biol. 2017 Aug 1;428(1):101-117. doi: 10.1016/j.ydbio.2017.05.020. Epub 2017 May 25. Dev Biol. 2017. PMID: 28551285
-
Transcriptome landscapes that signify Botrylloides leachi (Ascidiacea) torpor states.Dev Biol. 2022 Oct;490:22-36. doi: 10.1016/j.ydbio.2022.06.005. Epub 2022 Jul 7. Dev Biol. 2022. PMID: 35809632
-
Microbiome Restructuring: Dominant Coral Bacterium Endozoicomonas Species Respond Differentially to Environmental Changes.mSystems. 2022 Aug 30;7(4):e0035922. doi: 10.1128/msystems.00359-22. Epub 2022 Jun 15. mSystems. 2022. PMID: 35703535 Free PMC article.
-
Potential role of the gut microbiota in synthetic torpor and therapeutic hypothermia.World J Gastroenterol. 2017 Jan 21;23(3):406-413. doi: 10.3748/wjg.v23.i3.406. World J Gastroenterol. 2017. PMID: 28210076 Free PMC article. Review.
-
Whole-Body Regeneration in the Colonial Tunicate Botrylloides leachii.Results Probl Cell Differ. 2018;65:337-355. doi: 10.1007/978-3-319-92486-1_16. Results Probl Cell Differ. 2018. PMID: 30083927 Review.
Cited by
-
Effects of color variation and physiological state on ascidian microbiomes.Microbiologyopen. 2024 Apr;13(2):e1405. doi: 10.1002/mbo3.1405. Microbiologyopen. 2024. PMID: 38481089 Free PMC article.
References
-
- Benes V., Blake J., Doyle K. (2011). Ribo-Zero Gold Kit: improved RNA-seq results after removal of cytoplasmic and mitochondrial ribosomal RNA. Nat. Methods 8, iii–iv. doi: 10.1038/nmeth.f.352 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

