Calcium Supplementation Ameliorates Cerebellar Oxidative Stress in Lactational Aluminum-induced Neurotoxicity in Rats
- PMID: 37323952
- PMCID: PMC10262293
- DOI: 10.32598/bcn.2022.1347.2
Calcium Supplementation Ameliorates Cerebellar Oxidative Stress in Lactational Aluminum-induced Neurotoxicity in Rats
Abstract
Introduction: The neurotoxic effects of aluminum exposure during the critical period of neurodevelopment have been well documented. This study investigated the known protective effects of calcium supplementation on the cerebellum of juvenile Wistar rats following aluminum-induced neurotoxicity during lactation.
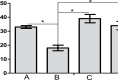
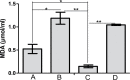
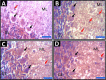
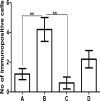
Methods: Four groups of juvenile rats were exposed via lactation to distilled water (control group), aluminum (40 mg/kg/d), calcium supplement (50 mg/kg/d), and a combination of both aluminum and calcium from postnatal day 4 to day 28. The cerebella of the animals were excised to access the levels of antioxidant enzymes (superoxide dismutase [SOD], glutathione peroxidase [GPx]), lipid peroxidation (malondialdehyde), histomorphological alterations (hematoxylin and eosin staining), Nissl profile (cresyl fast violet staining), and glial activation (glial fibrillary acidic protein immunohistochemistry).
Results: Lactational aluminum significantly decreased the activities of superoxide dismutase and glutathione peroxidase while exacerbating lipid peroxidation and reactive astrocyte in cerebellar lysates. Lactational calcium supplementation normalized the activities of SOD and GPx, thereby preventing excessive lipid peroxidation and glial activation. Despite no apparent changes in the general histology of the cerebellum, aluminum-induced chromatolysis changes in the Purkinje cell layer, which was counteracted by the antioxidant propensities of calcium supplementation.
Conclusion: These findings support that calcium supplementation significantly protects the cerebellum against aluminum-induced oxidative stress, chromatolysis, and neuroinflammation.
Keywords: Aluminum; Astroglia; Calcium; Cerebellum; Glial activation; Lactation; Oxidative stress.
Copyright© 2022 Iranian Neuroscience Society.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest.
Figures


Similar articles
-
Thalamic superoxide and peroxide handling capacity (SPHC): An experimental study with aluminum, ethanol and tocopherol in rats.Indian J Exp Biol. 2015 Sep;53(9):568-73. Indian J Exp Biol. 2015. PMID: 26548076
-
Histomorphological evaluations on the frontal cortex extrapyramidal cell layer following administration of N-Acetyl cysteine in aluminum induced neurodegeneration rat model.Metab Brain Dis. 2020 Jun;35(5):829-839. doi: 10.1007/s11011-020-00556-9. Epub 2020 Mar 24. Metab Brain Dis. 2020. PMID: 32212044 Free PMC article.
-
Perinatal exposure to lead: reduction in alterations of brain mitochondrial antioxidant system with calcium supplement.Biol Trace Elem Res. 2014 Dec;162(1-3):270-7. doi: 10.1007/s12011-014-0112-7. Epub 2014 Aug 28. Biol Trace Elem Res. 2014. PMID: 25161091
-
Melatonin ameliorates oxidative damage induced by maternal lead exposure in rat pups.Physiol Behav. 2015 Nov 1;151:178-88. doi: 10.1016/j.physbeh.2015.06.040. Epub 2015 Jul 18. Physiol Behav. 2015. PMID: 26197271
-
Postnatal Administration of Homocysteine Induces Cerebellar Damage in Rats: Protective Effect of Folic Acid.Neurotox Res. 2019 Apr;35(3):724-738. doi: 10.1007/s12640-018-9979-y. Epub 2018 Nov 15. Neurotox Res. 2019. PMID: 30443710
References
-
- Ahmad P., Abdel Latef A. A., Abd_Allah E. F., Hashem A., Sarwat M., Anjum N. A., et al. (2016). Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed Chickpea (Cicer arietinum L.). Frontiers in Plant Science, 7, 513. [DOI:10.3389/fpls.2016.00513] - DOI - PMC - PubMed
-
- Berchner-Pfannschmidt U., Petrat F., Doege K., Trinidad B., Freitag P., Metzen E., et al. (2004). Chelation of cellular calcium modulates hypoxia-inducible gene expression through activation of hypoxia-inducible factor-1α. The Journal of Biological Chemistry, 279(43), 44976–44986. [DOI:10.1074/jbc.M313995200] [PMID] - DOI - PubMed
LinkOut - more resources
Full Text Sources
