Immunotherapy-related adverse events in real-world patients with advanced non-small cell lung cancer on chemoimmunotherapy: a Spinnaker study sub-analysis
- PMID: 37324003
- PMCID: PMC10265987
- DOI: 10.3389/fonc.2023.1163768
Immunotherapy-related adverse events in real-world patients with advanced non-small cell lung cancer on chemoimmunotherapy: a Spinnaker study sub-analysis
Abstract
Background: The Spinnaker study evaluated survival outcomes and prognostic factors in patients with advanced non-small-cell lung cancer receiving first-line chemoimmunotherapy in the real world. This sub-analysis assessed the immunotherapy-related adverse effects (irAEs) seen in this cohort, their impact on overall survival (OS) and progression-free survival (PFS), and related clinical factors.
Methods: The Spinnaker study was a retrospective multicentre observational cohort study of patients treated with first-line pembrolizumab plus platinum-based chemotherapy in six United Kingdom and one Swiss oncology centres. Data were collected on patient characteristics, survival outcomes, frequency and severity of irAEs, and peripheral immune-inflammatory blood markers, including the neutrophil-to-lymphocyte ratio (NLR) and systemic immune-inflammation index (SII).
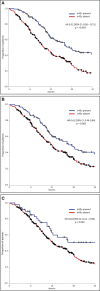
Results: A total of 308 patients were included; 132 (43%) experienced any grade irAE, 100 (32%) Grade 1-2, and 49 (16%) Grade 3-4 irAEs. The median OS in patients with any grade irAES was significantly longer (17.5 months [95% CI, 13.4-21.6 months]) than those without (10.1 months [95% CI, 8.3-12.0 months]) (p<0.001), either if Grade 1-2 (p=0.003) or Grade 3-4 irAEs (p=0.042). The median PFS in patients with any grade irAEs was significantly longer (10.1 months [95% CI, 9.0-11.2 months]) than those without (6.1 months [95% CI, 5.2-7.1 months]) (p<0.001), either if Grade 1-2 (p=0.011) or Grade 3-4 irAEs (p=0.036). A higher rate of irAEs of any grade and specifically Grade 1-2 irAEs correlated with NLR <4 (p=0.013 and p=0.018), SII <1,440 (p=0.029 ad p=0.039), response to treatment (p=0.001 and p=0.034), a higher rate of treatment discontinuation (p<0.00001 and p=0.041), and the NHS-Lung prognostic classes (p=0.002 and p=0.008).
Conclusions: These results confirm survival outcome benefits in patients with irAEs and suggest a higher likelihood of Grade 1-2 irAEs in patients with lower NLR or SII values or according to the NHS-Lung score.
Keywords: immune-related adverse effects; immunotherapy; lung cancer; neutrophil-to-lymphocyte ratio (NLR); non-small cell lung cancer; overall survival; progression free survival; systemic immune-inflammation index (SII).
Copyright © 2023 Anpalakhan, Huddar, Behrouzi, Signori, Cave, Comins, Cortellini, Addeo, Escriu, McKenzie, Barone, Murray, Pinato, Ottensmeier, Campos, Muthuramalingam, Chan, Gomes and Banna.
Conflict of interest statement
GB received grant consultancies from Astrazeneca and Astellas Pharma. AC received speaker fees and grant consultancies by Astrazeneca, MSD, IQVIA, OncoC4, and EISAI. AA received consulting fees from BMS, Astrazeneca, Boehringer-Ingelheim, Roche, MSD, Pfizer, Eli Lilly, and Astellas, and speakers fees from Eli Lilly and Astrazeneca. DP received lecture fees from ViiV Healthcare, Bayer Healthcare, BMS, Roche, EISAI, and Falk Foundation; travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, Avamune, Exact Sciences, Mursla, DaVolterra, and Astra Zeneca; and research funding to institution from MSD and BMS. GB received travel expenses from Novartis. CO reports personal fees from BMS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Mazieres J, Rittmeyer A, Gadgeel S, Hida T, Gandara DR, Cortinovis DL, et al. . Atezolizumab versus docetaxel in pretreated patients with NSCLC: final results from the randomized phase 2 POPLAR and phase 3 OAK clinical trials. J Thorac Oncol (2021) 16:140–50. doi: 10.1016/j.jtho.2020.09.022 - DOI - PubMed
LinkOut - more resources
Full Text Sources