Predictors associated with mortality of veno-venous extracorporeal membrane oxygenation therapy
- PMID: 37324096
- PMCID: PMC10267924
- DOI: 10.21037/jtd-22-1273
Predictors associated with mortality of veno-venous extracorporeal membrane oxygenation therapy
Abstract
Background: The use of veno-venous extracorporeal membrane oxygenation (V-V ECMO) has rapidly increased in recent years. Today, applications of V-V ECMO include a variety of clinical conditions such as acute respiratory distress syndrome (ARDS), bridge to lung transplantation and primary graft dysfunction after lung transplantation. The purpose of the present study was to investigate in-hospital mortality of adult patients undergoing V-V ECMO therapy and to determine independent predictors associated with mortality.
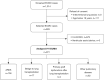
Methods: This retrospective study was conducted at the University Hospital Zurich, a designated ECMO center in Switzerland. Data was analyzed of all adult V-V ECMO cases from 2007 to 2019.
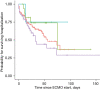
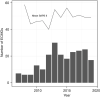
Results: In total, 221 patients required V-V ECMO support (median age 50 years, 38.9% female). In-hospital mortality was 37.6% and did not statistically vary significantly between indications (P=0.61): 25.0% (1/4) for primary graft dysfunction after lung transplantation, 29.4% (5/17) for bridge to lung transplantation, 36.2% (50/138) for ARDS and 43.5% (27/62) for other pulmonary disease indications. Cubic spline interpolation showed no effect of time on mortality over the study period of 13 years. Multiple logistic regression modelling identified significant predictor variables associated with mortality: age [odds ratio (OR), 1.05; 95% confidence interval (CI): 1.02-1.07; P=0.001], newly detected liver failure (OR, 4.83; 95% CI: 1.27-20.3; P=0.02), red blood cell transfusion (OR, 1.91; 95% CI: 1.39-2.74; P<0.001) and platelet concentrate transfusion (OR, 1.93; 95% CI: 1.28-3.15; P=0.004).
Conclusions: In-hospital mortality of patients receiving V-V ECMO therapy remains relatively high. Patients' outcomes have not improved significantly in the observed period. We identified age, newly detected liver failure, red blood cell transfusion and platelet concentrate transfusion as independent predictors associated with in-hospital mortality. Incorporating such mortality predictors into decision making with regards to V-V ECMO use may increase its effectiveness and safety and may translate into better outcomes.
Keywords: Extracorporeal membrane oxygenation (ECMO); mortality; outcome; predictors.
2023 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1273/coif). AK has received support from Bayer AG (Switzerland) for lecturing. DRS’ academic department is receiving grant support from the Swiss National Science Foundation, Berne, Switzerland, the Swiss Society of Anesthesiology and Perioperative Medicine (SSAPM), Berne, Switzerland, the Swiss Foundation for Anesthesia Research, Zurich, Switzerland, Vifor SA, Villars-sur-Glâne, Switzerland and Vifor (International) AG, St. Gallen, Switzerland. DRS is co-chair of the ABC-Trauma Faculty, sponsored by unrestricted educational grants from Novo Nordisk Health Care AG, Zurich, Switzerland, CSL Behring GmbH, Marburg, Germany, LFB Biomédicaments, Courtaboeuf Cedex, France and Octapharma AG, Lachen, Switzerland. DRS received honoraria/travel support for consulting or lecturing from: Alliance Rouge, Bern, Switzerland, Danube University of Krems, Austria, European Society of Anesthesiology and Intensive Care, Brussels, BE, International Foundation for Patient Blood Management, Basel, Switzerland, Korean Society of Anesthesiologists, Seoul, Korea, Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis, Paris, France, Society for the Advancement of Blood Management, Mount Royal NJ, Alexion Pharmaceuticals Inc., Boston, MA, AstraZeneca AG, Baar, Switzerland, Bayer AG, Zürich, Switzerland, B. Braun Melsungen AG, Melsungen, Germany, Baxter AG, Glattpark, Switzerland, CSL Behring GmbH, Hattersheim am Main, Germany and Berne, Switzerland, CSL Vifor (Switzerland) Villars-sur-Glâne, Switzerland, CSL Vifor (International), St. Gallen, Switzerland, Celgene International II Sàrl, Couvet, Switzerland, Daiichi Sankyo AG, Thalwil, Switzerland, Haemonetics, Braintree, MA, USA, LFB Biomédicaments, Courtaboeuf Cedex, France, Merck Sharp & Dohme, Kenilworth, New Jersey, USA, Novo Nordisk Health Care AG, Zurich, Switzerland, Octapharma AG, Lachen, Switzerland, Pharmacosmos A/S, Holbaek, Denmark, Pierre Fabre Pharma, Alschwil, Switzerland, Portola Schweiz GmbH, Aarau, Switzerland, Roche Diagnostics International Ltd, Reinach, Switzerland, Sarstedt AG & Co., Sevelen, Switzerland and Nümbrecht, Germany, Shire Switzerland GmbH, Zug, Switzerland, Takeda, Glattpark, Switzerland, Werfen, Bedford, MA, Zuellig Pharma Holdings, Singapore, Singapore. MJW received speaker’s honoraria and reimbursement for travel expenses from Berlin Heart GmbH, Berlin, Germany, not related to this article. The other authors have no conflicts of interest to declare.
Figures

References
LinkOut - more resources
Full Text Sources
