Spatial characterization of tangle-bearing neurons and ghost tangles in the human inferior temporal gyrus with three-dimensional imaging
- PMID: 37324243
- PMCID: PMC10263274
- DOI: 10.1093/braincomms/fcad130
Spatial characterization of tangle-bearing neurons and ghost tangles in the human inferior temporal gyrus with three-dimensional imaging
Abstract
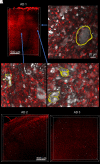
Studies of post-mortem human tissue provide insight into pathological processes, but are inherently limited by practical considerations that limit the scale at which tissue can be examined, and the obvious issue that the tissue reflects only one time point in a continuous disease process. We approached this problem by adapting new tissue clearance techniques to an entire cortical area of human brain, which allows surveillance of hundreds of thousands of neurons throughout the depth of the entire cortical thickness. This approach allows detection of 'rare' events that may be difficult to detect in standard 5 micrometre-thick paraffin sections. For example, it is well established that neurofibrillary tangles begin within a neuron, and ultimately, in at least some instances, persist in the brain even after the neuron has died. These are referred to as 'ghost tangles', a term that appropriately implies their 'difficult to see' ephemeral qualities. We set out to find ghost tangles as one example of the power of the tissue clearance/image analysis techniques to detect rare events, and to learn what happens at the end-point of a tangle's life history. We were able to identify 8103 tau tangles, 132 465 neurons and 299 640 nuclei in tissue samples from three subjects with severe Alzheimer's disease (Braak V-VI) and 4 tau tangles, 200 447 neurons and 462 715 nuclei in tissue samples from three subjects with no significant tau pathology (Braak 0-I). Among these data, we located 57 ghost tangles, which makes them only 0.7% of the total tau tangles observed. We found that ghost tangles are more likely to be found in cortical layers 3 and 5 (49/57), with a select few scattered across other layers 1, 2, 4 and 6. This ability to find rare events, such as ghost tangles, in large enough quantities to statistically test their distribution exemplifies how tissue clearing can be used as a powerful tool for studying selective vulnerability or resilience to pathology across brain regions.
Keywords: ghost tangles; inferior temporal gyrus; spatial mapping; tau; tissue clearing.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors declare no competing interests. Dr. Hyman has a family member who works for Novartis and has equity and serves on the Senior Advisory Boards of Dewpoint and Lattus.
Figures




References
-
- Rusch H, Brammerloh M, Stieler J, et al. Finding the best clearing approach—Towards 3D wide-scale multimodal imaging of aged human brain tissue. Neuroimage. 2022;247:118832. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
