Satellite cell contribution to disease pathology in Duchenne muscular dystrophy
- PMID: 37324396
- PMCID: PMC10266354
- DOI: 10.3389/fphys.2023.1180980
Satellite cell contribution to disease pathology in Duchenne muscular dystrophy
Abstract
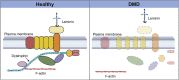
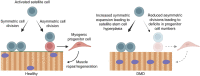
Progressive muscle weakness and degeneration characterize Duchenne muscular dystrophy (DMD), a lethal, x-linked neuromuscular disorder that affects 1 in 5,000 boys. Loss of dystrophin protein leads to recurrent muscle degeneration, progressive fibrosis, chronic inflammation, and dysfunction of skeletal muscle resident stem cells, called satellite cells. Unfortunately, there is currently no cure for DMD. In this mini review, we discuss how satellite cells in dystrophic muscle are functionally impaired, and how this contributes to the DMD pathology, and the tremendous potential of restoring endogenous satellite cell function as a viable treatment strategy to treat this debilitating and fatal disease.
Keywords: Duchenne muscular dystrophy; asymmetric cell division; dystrophin; muscle regeneration; myogenesis; satellite cells; skeletal muscle stem cells; symmetric cell division.
Copyright © 2023 Kodippili and Rudnicki.
Conflict of interest statement
MR is a Founding Scientist and CSO of Satellos Bioscience, and KK is supported from a research contract from Satellos Bioscience.
Figures

References
-
- Aartsma-Rus A., Van Deutekom J. C., Fokkema I. F., Van Ommen G. J., Den Dunnen J. T. (2006). Entries in the leiden duchenne muscular dystrophy mutation database: An overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 34, 135–144. 10.1002/mus.20586 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

