Hydroxychloroquine-induced Retinal Toxicity
- PMID: 37324471
- PMCID: PMC10267834
- DOI: 10.3389/fphar.2023.1196783
Hydroxychloroquine-induced Retinal Toxicity
Abstract
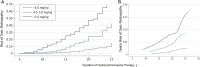
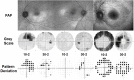
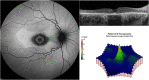
Long-term use of hydroxychloroquine can cause retinopathy, which may result in severe and progressive visual loss. In the past decade, hydroxychloroquine use has markedly increased and modern retinal imaging techniques have enabled the detection of early, pre-symptomatic disease. As a consequence, the prevalence of retinal toxicity in long-term hydroxychloroquine users is known to be higher than was previously estimated. The pathophysiology of the retinopathy is incompletely characterised, although significant advances have been made in understanding the disease from clinical imaging studies. Hydroxychloroquine retinopathy elicits sufficient public health concern to justify the implementation of retinopathy screening programs for patients at risk. Here, we describe the historical background of hydroxychloroquine retinopathy and summarize its current understanding. We review the utility and limitations of each of the mainstream diagnostic tests used to detect hydroxychloroquine retinopathy. The key considerations towards a consensus on the definition of hydroxychloroquine retinopathy are outlined in the context of what is known of the natural history of the disease. We compare the current screening recommendations for hydroxychloroquine retinopathy, identifying where additional evidence is required, and the management of proven cases of toxicity. Finally, we highlight the areas for further investigation, which may further reduce the risk of visual loss in hydroxychloroquine users.
Keywords: hydroxychloroquine; hydroxychloroquine retinopathy; optical coherence tomography; retinal imaging; retinopathy; screening; toxicity.
Copyright © 2023 Yusuf, Charbel Issa and Ahn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

