CX3CL1/CX3CR1 axis alleviates inflammation and apoptosis in human nucleus pulpous cells via M2 macrophage polarization
- PMID: 37324510
- PMCID: PMC10265713
- DOI: 10.3892/etm.2023.12058
CX3CL1/CX3CR1 axis alleviates inflammation and apoptosis in human nucleus pulpous cells via M2 macrophage polarization
Abstract
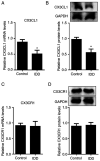
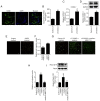
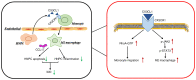
CX3C chemokine ligand 1 (CX3CL1) belongs to the CX3C chemokine family and is involved in various disease processes. However, its role in intervertebral disc degeneration (IDD) remains to be elucidated. In the present study, western blotting, reverse transcription-quantitative PCR and ELISA assays were used to assess target gene expression. In addition, immunofluorescence and TUNEL staining were used to assess macrophage infiltration, monocyte migration and apoptosis. The present study aimed to reveal if and how CX3CL1 regulates IDD progression by exploring its effect on macrophage polarization and apoptosis of human nucleus pulposus cells (HNPCs). The data showed that CX3CL1 bound to CX3C motif chemokine receptor 1 (CX3CR1) promoted the M2 phenotype polarization via JAK2/STAT3 signaling, followed by increasing the secretion of anti-inflammatory cytokines from HNPCs. In addition, HNPC-derived CX3CL1 promoted M2 macrophage-derived C-C motif chemokine ligand 17 release thereby reducing the apoptosis of HNPCs. In clinic, the reduction of mRNA and protein levels CX3CL1 in degenerative nucleus pulposus tissues (NPs) was measured. Increased M1 macrophages and pro-inflammatory cytokines were found in NPs of IDD patients with low CX3CL1 expression. Collectively, these findings suggested that the CX3CL1/CX3CR1 axis alleviates IDD by reducing inflammation and apoptosis of HNPCs via macrophages. Therefore, targeting CX3CL1/CX3CR1 axis is expected to produce a new therapeutic approach for IDD.
Keywords: CX3C chemokine ligand 1; CX3C motif chemokine receptor 1; apoptosis; inflammation; intervertebral disc degeneration; macrophage.
Copyright: © Gao et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
CX3CL1/CX3CR1 interaction protects against lipotoxicity-induced nonalcoholic steatohepatitis by regulating macrophage migration and M1/M2 status.Metabolism. 2022 Nov;136:155272. doi: 10.1016/j.metabol.2022.155272. Epub 2022 Jul 29. Metabolism. 2022. PMID: 35914622
-
Myeloid-derived growth factor promotes M2 macrophage polarization and attenuates Sjögren's syndrome via suppression of the CX3CL1/CX3CR1 axis.Front Immunol. 2024 Oct 21;15:1465938. doi: 10.3389/fimmu.2024.1465938. eCollection 2024. Front Immunol. 2024. PMID: 39497829 Free PMC article.
-
CX3CL1 deficiency ameliorates inflammation, apoptosis and accelerates osteogenic differentiation, mineralization in LPS-treated MC3T3-E1 cells via its receptor CX3CR1.Ann Anat. 2023 Feb;246:152036. doi: 10.1016/j.aanat.2022.152036. Epub 2022 Nov 24. Ann Anat. 2023. PMID: 36436718
-
CX3C chemokine: Hallmarks of fibrosis and ageing.Pharmacol Res. 2024 Oct;208:107348. doi: 10.1016/j.phrs.2024.107348. Epub 2024 Aug 10. Pharmacol Res. 2024. PMID: 39134186 Review.
-
Structure and Function of Ligand CX3CL1 and its Receptor CX3CR1 in Cancer.Curr Med Chem. 2022;29(41):6228-6246. doi: 10.2174/0929867329666220629140540. Curr Med Chem. 2022. PMID: 35770395 Review.
Cited by
-
Regulating macrophage phenotypes with IL4I1-mimetic nanoparticles in IDD treatment.J Nanobiotechnology. 2025 Mar 6;23(1):175. doi: 10.1186/s12951-025-03241-0. J Nanobiotechnology. 2025. PMID: 40050923 Free PMC article.
-
Role of macrophage in intervertebral disc degeneration.Bone Res. 2025 Jan 23;13(1):15. doi: 10.1038/s41413-024-00397-7. Bone Res. 2025. PMID: 39848963 Free PMC article. Review.
-
Molecular dynamics of inflammation resolution: therapeutic implications.Front Cell Dev Biol. 2025 May 8;13:1600149. doi: 10.3389/fcell.2025.1600149. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40406415 Free PMC article. Review.
-
Analysis of global research hotspots and trends in immune cells in intervertebral disc degeneration: A bibliometric study.Hum Vaccin Immunother. 2023 Dec 15;19(3):2274220. doi: 10.1080/21645515.2023.2274220. Epub 2023 Nov 9. Hum Vaccin Immunother. 2023. PMID: 37941392 Free PMC article.
-
CX3CL1 (Fractalkine)-CX3CR1 Axis in Inflammation-Induced Angiogenesis and Tumorigenesis.Int J Mol Sci. 2024 Apr 25;25(9):4679. doi: 10.3390/ijms25094679. Int J Mol Sci. 2024. PMID: 38731899 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
