Biological fractionation of lithium isotopes by cellular Na+/H+ exchangers unravels fundamental transport mechanisms
- PMID: 37324528
- PMCID: PMC10265516
- DOI: 10.1016/j.isci.2023.106887
Biological fractionation of lithium isotopes by cellular Na+/H+ exchangers unravels fundamental transport mechanisms
Abstract
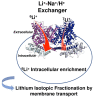
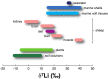
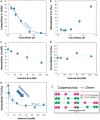
Lithium (Li) has a wide range of uses in science, medicine, and industry, but its isotopy is underexplored, except in nuclear science and in geoscience. 6Li and 7Li isotopic ratio exhibits the second largest variation on earth's surface and constitutes a widely used tool for reconstructing past oceans and climates. As large variations have been measured in mammalian organs, plants or marine species, and as 6Li elicits stronger effects than natural Li (∼95% 7Li), a central issue is the identification and quantification of biological influence of Li isotopes distribution. We show that membrane ion channels and Na+-Li+/H+ exchangers (NHEs) fractionate Li isotopes. This systematic 6Li enrichment is driven by membrane potential for channels, and by intracellular pH for NHEs, where it displays cooperativity, a hallmark of dimeric transport. Evidencing that transport proteins discriminate between isotopes differing by one neutron opens new avenues for transport mechanisms, Li physiology, and paleoenvironments.
Keywords: Biochemistry; Biological sciences; Isotope chemistry.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Balter V., Vigier N. Natural variations of lithium isotopes in a mammalian model. Metallomics. 2014;6:582–586. - PubMed
-
- Burton K.W., Vigier N. Handbook of Environmental Isotope Geochemistry. Vol. 1. 2011. Lithium isotopes as tracers in marine and terrestrial environments Springer-Verlag; pp. 41–60.
-
- Penniston-Dorland S., Liu X.-M., Rudnick R.L. Lithium isotope geochemistry. Rev. Mineral. Geochem. 2017;82:165–217.
-
- Roberts J., Kaczmarek K., Langer G., Skinner L.C., Bijma J., Bradbury H., Turchyn A., Lamy F., Misra S. Lithium isotopic composition of benthic foraminifera: a new proxy for paleo-pH reconstruction Geochim. Cosmochim. Acta. 2018;236:336–350.
-
- Vigier N., Rollion-Bard C., Levenson Y., Erez J. Lithium isotopes in foraminifera shells as a novel proxy for the ocean dissolved inorganic carbon (DIC) C. R. Geosci. 2015;347:43–51.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

