Solvent-in-Salt Electrolytes for Fluoride Ion Batteries
- PMID: 37324537
- PMCID: PMC10262201
- DOI: 10.1021/acsenergylett.3c00493
Solvent-in-Salt Electrolytes for Fluoride Ion Batteries
Abstract
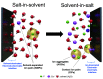
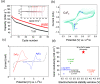
The fluoride ion battery (FIB) is a promising post-lithium ion battery chemistry owing to its high theoretical energy density and the large elemental abundance of its active materials. Nevertheless, its utilization for room-temperature cycling has been impeded by the inability to find sufficiently stable and conductive electrolytes at room temperature. In this work, we report the use of solvent-in-salt electrolytes for FIBs, exploring multiple solvents to show that aqueous cesium fluoride exhibited sufficiently high solubility to achieve an enhanced (electro)chemical stability window (3.1 V) that could enable high operating voltage electrodes, in addition to a suppression of active material dissolution that allows for an improved cycling stability. The solvation structure and transport properties of the electrolyte are also investigated using spectroscopic and computational methods.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Choi J. W.; Aurbach D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013.10.1038/natrevmats.2016.13. - DOI
-
- Anji Reddy M.; Fichtner M. Batteries based on fluoride shuttle. J. Mater. Chem. 2011, 21, 17059–17062. 10.1039/c1jm13535j. - DOI
-
- Xiao A. W.; Galatolo G.; Pasta M. The case for fluoride-ion batteries. Joule 2021, 5, 2823–2844. 10.1016/j.joule.2021.09.016. - DOI
-
- Nowroozi M. A.; Mohammad I.; Molaiyan P.; Wissel K.; Munnangi A. R.; Clemens O. Fluoride ion batteries - past, present, and future. J. Mater. Chem. A 2021, 9, 5980–6012. 10.1039/D0TA11656D. - DOI
LinkOut - more resources
Full Text Sources
