Agrobacterium-mediated direct transformation of wheat mature embryos through organogenesis
- PMID: 37324676
- PMCID: PMC10264787
- DOI: 10.3389/fpls.2023.1202235
Agrobacterium-mediated direct transformation of wheat mature embryos through organogenesis
Abstract
Transgenic plant production in monocotyledonous species has primarily relied on embryogenic callus induction from both immature and mature embryos as the pathway for plant regeneration. We have efficiently regenerated fertile transgenic wheat plants through organogenesis after Agrobacterium-mediated direct transformation of mechanically isolated mature embryos from field-grown seed. Centrifugation of the mature embryos in the presence of Agrobacterium was found to be essential for efficient T-DNA delivery to the relevant regenerable cells. The inoculated mature embryos formed multiple buds/shoots on high-cytokinin medium, which directly regenerated into transgenic shoots on hormone-free medium containing glyphosate for selection. Rooted transgenic plantlets were obtained within 10-12 weeks after inoculation. Further optimization of this transformation protocol resulted in significant reduction of chimeric plants to below 5%, as indicated by leaf GUS staining and T1 transgene segregation analysis. Direct transformation of wheat mature embryos has substantial advantages over traditional immature embryo-based transformation systems, including long-term storability of the mature dry explants, scalability, and greatly improved flexibility and consistency in transformation experiments.
Keywords: agrobacterium-mediated transformation; centrifugation-assisted Agrobacterium inoculation; mature embryo; multiple buds; multiple shoots; organogenesis; transgenic plant; wheat.
Copyright © 2023 Ye, Shrawat, Moeller, Rode, Rivlin, Kelm, Martinell, Williams, Paisley, Duncan and Armstrong.
Conflict of interest statement
The research activities in this report were conducted by teams at legacy Monsanto Company, now Bayer Crop Science, a manufacturer of seeds produced by conventional and biotechnology methods. The authors contributed to this research as employees. A relevant US patent application has been filed and assigned to Bayer Crop Science.
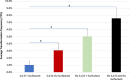
Figures






References
-
- Aadel H., Abdelwahd R., Udupa S. M., Diria G., El Mouhtadi A., Ahansal K., et al. . (2018). Agrobacterium-mediated transformation of mature embryo tissues of bread wheat (Triticum aestivum l.) genotypes. Cereal Res. Commun. 46, 10–20. doi: 10.1556/0806.45.2017.055 - DOI
-
- Chen Y., Rivlin A., Lange A., Ye X., Vaghchhipawala Z., Eisinger E., et al. . (2014). High throughput Agrobacterium tumefaciens-mediated germline transformation of mechanically isolated meristem explants of cotton (Gossypium hirsutum l.). Plant Cell Rep. 33, 153–164. doi: 10.1007/s00299-013-1519-x - DOI - PubMed
-
- Dellaporta S. L., Wood J., Hicks J. B. (1983). A plant DNA minipreparation: version II. Plant Mol. Biol. Rep. 1, 19–21. doi: 10.1007/BF02712670 - DOI
LinkOut - more resources
Full Text Sources

