Cardiac implantable electronic devices (CIEDs) and allergy
- PMID: 37324770
- PMCID: PMC10264757
- DOI: 10.1002/joa3.12852
Cardiac implantable electronic devices (CIEDs) and allergy
Abstract
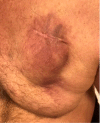
Advances in cardiac implantable electronic devices (CIEDs) have prolonged life expectancy in various medical settings. However, the issue of hypersensitivity to components of CIEDs is still a concern. Since 1970, allergic reactions to metallic and nonmetallic components of CIEDs have been reported. Hypersensitivity reactions to medical devices are rare and not fully understood. In some cases, diagnosis and treatment are difficult. Cardiologists should always keep in mind pacemaker allergy when a patient appears with wound complications and no signs of infection. Patch testing should be tailored toward the specific biomaterials used in a device, in addition to testing with standard screening allergens in select cases.
Keywords: allergic reactions; cardiac implantable electronic devices; hypersensitivity.
© 2023 The Authors. Journal of Arrhythmia published by John Wiley & Sons Australia, Ltd on behalf of Japanese Heart Rhythm Society.
Conflict of interest statement
No financial support was received and none of the authors has any conflict of interest.
Figures

References
-
- Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Europace. 2022;24(1):71–164. - PubMed
-
- Raatikainen MJ, Arnar DO, Merkely B, Camm AJ, Hindricks G. Access to and clinical use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology Countries: 2016 Report from the European Heart Rhythm Association. Europace. 2016;18(Suppl 3):iii1–iii79. - PubMed
-
- Ellenbogen KA, Hellkamp AS, Wilkoff BL, Camunas JL, Love JC, Hadjis TA, et al. Complications arising after implantation of DDD pacemakers: the MOST experience. Am J Cardiol. 2003;92:740–1. - PubMed
-
- Udo EO, Zuithoff NP, van Hemel NM, de Cock CC, Hendriks T, Doevendans PA, et al. Incidence and predictors of short‐ and long‐term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. 2012;9:728–35. - PubMed
-
- Parsonnet V, Bernstein AD, Lindsay B. Pacemaker‐implantation complication rates: an analysis of some contributing factors. J Am Coll Cardiol. 1989;13:917–21. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

