Ability of Local Clearance of Senescent Cells in Ipsilateral Hemisphere to Mitigate Acute Ischemic Brain Injury in Mice
- PMID: 37324944
- PMCID: PMC10266088
- DOI: 10.7150/ijbs.84060
Ability of Local Clearance of Senescent Cells in Ipsilateral Hemisphere to Mitigate Acute Ischemic Brain Injury in Mice
Abstract
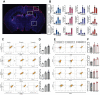
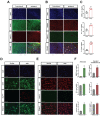
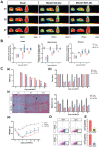
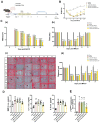
Senolytic treatment has potential therapeutic efficacy for acute ischemic stroke (AIS). However, the systemic treatment of senolytics may produce off-target side effects and a toxic profile, which affect analysis of the role of acute senescence of neuronal cells in pathogenesis of AIS. We constructed a novel lenti-INK-ATTAC viral vector to introduce INK-ATTAC genes to the ipsilateral brain and locally eliminate senescent brain cells by administering AP20187 to activate caspase-8 apoptotic cascade. In this study, we have found that acute senescence is triggered by middle cerebral artery occlusion (MCAO) surgery, particularly in astrocytes and cerebral endothelial cells (CECs). The upregulation of p16INK4a and senescence-associated secretory phenotype (SASP) factors including matrix metalloproteinase-3, interleukin-1 alpha and -6 were observed in oxygen-glucose deprivation-treated astrocytes and CECs. The systemic administration of a senolytic, ABT-263, prevented the impairment of brain activity from hypoxic brain injury in mice, and significantly improved the neurological severity score, rotarod performance, locomotor activity, and weight loss. The treatment of ABT-263 reduced senescence of astrocytes and CECs in MCAO mice. Furthermore, the localized removal of senescent cells in the injured brain through the stereotaxical injection of lenti-INK-ATTAC viruses generates neuroprotective effects, protecting against acute ischemic brain injury in mice. The content of SASP factors and mRNA level of p16INK4a in the brain tissue of MCAO mice were significantly reduced by the infection of lenti-INK-ATTAC viruses. These results indicate that local clearance of senescent brain cells is a potential therapy on AIS, and demonstrate the correlation between neuronal senescence and pathogenesis of AIS.
Keywords: acute ischemic stroke; lenti-INK-ATTAC viral vector; senolytic treatment.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Senescence and SASP Are Potential Therapeutic Targets for Ischemic Stroke.Pharmaceuticals (Basel). 2024 Feb 28;17(3):312. doi: 10.3390/ph17030312. Pharmaceuticals (Basel). 2024. PMID: 38543098 Free PMC article. Review.
-
Targeted clearance of p21- but not p16-positive senescent cells prevents radiation-induced osteoporosis and increased marrow adiposity.Aging Cell. 2022 May;21(5):e13602. doi: 10.1111/acel.13602. Epub 2022 Apr 1. Aging Cell. 2022. PMID: 35363946 Free PMC article.
-
Progressive Cellular Senescence Mediates Renal Dysfunction in Ischemic Nephropathy.J Am Soc Nephrol. 2021 Aug;32(8):1987-2004. doi: 10.1681/ASN.2020091373. Epub 2021 Jun 16. J Am Soc Nephrol. 2021. PMID: 34135081 Free PMC article.
-
Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice.Aging Cell. 2021 Feb;20(2):e13296. doi: 10.1111/acel.13296. Epub 2021 Jan 20. Aging Cell. 2021. PMID: 33470505 Free PMC article.
-
Senolytics: A Translational Bridge Between Cellular Senescence and Organismal Aging.Front Cell Dev Biol. 2020 Jan 22;7:367. doi: 10.3389/fcell.2019.00367. eCollection 2019. Front Cell Dev Biol. 2020. PMID: 32039197 Free PMC article. Review.
Cited by
-
Ischemic Stroke and the Biological Hallmarks of Aging.Aging Dis. 2024 Sep 30;16(5):2908-2936. doi: 10.14336/AD.2024.1059. Aging Dis. 2024. PMID: 40789569 Free PMC article. Review.
-
Senescence and SASP Are Potential Therapeutic Targets for Ischemic Stroke.Pharmaceuticals (Basel). 2024 Feb 28;17(3):312. doi: 10.3390/ph17030312. Pharmaceuticals (Basel). 2024. PMID: 38543098 Free PMC article. Review.
-
Anti-aging Factor GRSF1 Attenuates Cerebral Ischemia-Reperfusion Injury in Mice by Inhibiting GPX4-Mediated Ferroptosis.Mol Neurobiol. 2024 Apr;61(4):2151-2164. doi: 10.1007/s12035-023-03685-1. Epub 2023 Oct 20. Mol Neurobiol. 2024. PMID: 37861894
-
Therapeutic targeting of senescent cells in the CNS.Nat Rev Drug Discov. 2024 Nov;23(11):817-837. doi: 10.1038/s41573-024-01033-z. Epub 2024 Sep 30. Nat Rev Drug Discov. 2024. PMID: 39349637 Free PMC article. Review.
-
Senolytic treatment diminishes microglia and decreases severity of experimental autoimmune encephalomyelitis.J Neuroinflammation. 2024 Nov 1;21(1):283. doi: 10.1186/s12974-024-03278-2. J Neuroinflammation. 2024. PMID: 39487537 Free PMC article.
References
-
- Katan M, Luft A. Global burden of stroke. Seminars in neurology: Thieme Medical Publishers. 2018. p. 208-11. - PubMed
-
- Warlow CP, Van Gijn J, Dennis MS, Wardlaw JM, Bamford JM, Hankey GJ, et al. Stroke: practical management: John Wiley & Sons; 2011.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

