Starburst amacrine cells amplify optogenetic visual restoration through gap junctions
- PMID: 37324975
- PMCID: PMC10265492
- DOI: 10.1016/j.omtm.2023.05.011
Starburst amacrine cells amplify optogenetic visual restoration through gap junctions
Abstract
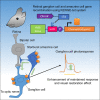
Ectopic induction of optogenetic actuators, such as channelrhodopsin, is a promising approach to restoring vision in the degenerating retina. However, the cell type-specific response of ectopic photoreception has not been well understood. There are limits to obtaining efficient gene expression in a specifically targeted cell population by a transgenic approach. In the present study, we established a murine model with high efficiency of gene induction to retinal ganglion cells (RGCs) and amacrine cells using an improved tetracycline transactivator-operator bipartite system (KENGE-tet system). To investigate the cell type-specific visual restorative effect, we expressed the channelrhodopsin gene into RGCs and amacrine cells using the KENGE-tet system. As a result, enhancement in the visual restorative effect was observed to RGCs and starburst amacrine cells. In conclusion, a photoresponse from amacrine cells may enhance the maintained response of RGCs and further increase or improve the visual restorative effect.
Keywords: gene therapy; optogenetics; retina; starburst amacrine cells; visual restoration.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Optogenetic targeting of AII amacrine cells restores retinal computations performed by the inner retina.Mol Ther Methods Clin Dev. 2023 Sep 13;31:101107. doi: 10.1016/j.omtm.2023.09.003. eCollection 2023 Dec 14. Mol Ther Methods Clin Dev. 2023. PMID: 37868206 Free PMC article.
-
Retinal Wave Patterns Are Governed by Mutual Excitation among Starburst Amacrine Cells and Drive the Refinement and Maintenance of Visual Circuits.J Neurosci. 2016 Mar 30;36(13):3871-86. doi: 10.1523/JNEUROSCI.3549-15.2016. J Neurosci. 2016. PMID: 27030771 Free PMC article.
-
A structural basis for omnidirectional connections between starburst amacrine cells and directionally selective ganglion cells in rabbit retina, with associated bipolar cells.Vis Neurosci. 2002 Mar-Apr;19(2):145-62. doi: 10.1017/s0952523802191139. Vis Neurosci. 2002. PMID: 12385627
-
On and off pathways through amacrine cells in mammalian retina: the synaptic connections of "starburst" amacrine cells.Vision Res. 1983;23(11):1265-79. doi: 10.1016/0042-6989(83)90102-5. Vision Res. 1983. PMID: 6362185 Review.
-
Biophysical mechanisms and essential topography of directionally selective subunits in rabbit's retina.J Integr Neurosci. 2005 Sep;4(3):341-61. doi: 10.1142/s0219635205000860. J Integr Neurosci. 2005. PMID: 16178062 Review.
Cited by
-
Tropism of the AAV6.2 Vector in the Murine Retina.Int J Mol Sci. 2025 Feb 13;26(4):1580. doi: 10.3390/ijms26041580. Int J Mol Sci. 2025. PMID: 40004046 Free PMC article.
References
-
- Doroudchi M.M., Greenberg K.P., Liu J., Silka K.A., Boyden E.S., Lockridge J.A., Arman A.C., Janani R., Boye S.L., Boye S.E., et al. Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol. Ther. 2011;19:1220–1229. - PMC - PubMed
-
- Lagali P.S., Balya D., Awatramani G.B., Münch T.A., Kim D.S., Busskamp V., Cepko C.L., Roska B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat. Neurosci. 2008;11:667–675. - PubMed
-
- Cronin T., Vandenberghe L.H., Hantz P., Juttner J., Reimann A., Kacsó A.E., Huckfeldt R.M., Busskamp V., Kohler H., Lagali P.S., et al. Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol. Med. 2014;6:1175–1190. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

