Proline-rich acidic protein 1 upregulates mitotic arrest deficient 1 to promote cisplatin-resistance of colorectal carcinoma by restraining mitotic checkpoint complex assembly
- PMID: 37325046
- PMCID: PMC10266255
- DOI: 10.7150/jca.84048
Proline-rich acidic protein 1 upregulates mitotic arrest deficient 1 to promote cisplatin-resistance of colorectal carcinoma by restraining mitotic checkpoint complex assembly
Abstract
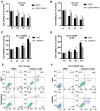
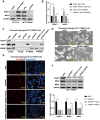
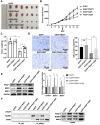
Background: The mechanism underlying cisplatin resistance in colorectal carcinoma (CRC) has not yet been elucidated. This study is aimed to illustrate the indispensable role of proline-rich acidic protein 1 (PRAP1) in cisplatin-resistant CRC. Methods: Cell viability and apoptosis were monitored using cell counting kit-8 and flow cytometry. Immunofluorescence and morphological analysis were used to determine mitotic arrest in cells. In vivo drug resistance was evaluated using a tumor xenograft assay. Results: PRAP1 was highly expressed in cisplatin-resistant CRC. PRAP1-upregulation in HCT-116 cells increased chemoresistance to cisplatin, whereas RNAi-mediated knockdown of PRAP1 sensitized cisplatin-resistant HCT-116 cells (HCT-116/DDP) to cisplatin. PRAP1-upregulation in HCT-116 cells hindered mitotic arrest and the formation of mitotic checkpoint complexes (MCC), followed by an increase in multidrug-resistant proteins such as p-glycoprotein 1 and multidrug resistance-associated protein 1, while PRAP1-knockdown in HCT-116/DDP cells partly restored colcemid-induced mitotic arrest and MCC assembly, resulting in decreased multidrug-resistant protein levels. PRAP1 downregulation-mediated sensitization to cisplatin in HCT-116/DDP cells was abolished by the inhibition of mitotic kinase activity by limiting MCC assembly. Additionally, PRAP1-upregulation increased cisplatin-resistance in CRC in vivo. Mechanistically, PRAP1 increased the expression of mitotic arrest deficient 1 (MAD1), that competitively binds to mitotic arrest deficient 2 (MAD2) in cisplatin-resistant CRC cells, leading to failed assembly of MCC and subsequent chemotherapy resistance. Conclusion: PRAP1-overexpression caused cisplatin resistance in CRC. Possibly, PRAP1 induced an increase in MAD1, which competitively interacted with MAD2 and subsequently restrained the formation of MCC, resulting in CRC cells escape from the supervision of MCC and chemotherapy resistance.
Keywords: Proline-rich acidic protein 1; cisplatin resistance; colorectal carcinoma; mitotic arrest deficient 1; spindle assembly checkpoint.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–20. - PubMed
-
- Brody H. Colorectal cancer. Nature. 2015;521:S1. - PubMed
-
- Teixeira C, Martins C, Dantas E, Trabulo D, Mangualde J, Freire R. et al. Interval colorectal cancer after colonoscopy. Rev Gastroenterol Mex (Engl Ed) 2019;84:284–9. - PubMed
-
- Wieszczy P, Kaminski MF, Franczyk R, Loberg M, Kobiela J, Rupinska M. et al. Colorectal Cancer Incidence and Mortality After Removal of Adenomas During Screening Colonoscopies. Gastroenterology. 2020;158:875–83.e5. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

