Obesity-Induced Coronary Microvascular Disease Is Prevented by iNOS Deletion and Reversed by iNOS Inhibition
- PMID: 37325396
- PMCID: PMC10264569
- DOI: 10.1016/j.jacbts.2022.11.005
Obesity-Induced Coronary Microvascular Disease Is Prevented by iNOS Deletion and Reversed by iNOS Inhibition
Abstract
Coronary microvascular disease (CMD) caused by obesity and diabetes is major contributor to heart failure with preserved ejection fraction; however, the mechanisms underlying CMD are not well understood. Using cardiac magnetic resonance applied to mice fed a high-fat, high-sucrose diet as a model of CMD, we elucidated the role of inducible nitric oxide synthase (iNOS) and 1400W, an iNOS antagonist, in CMD. Global iNOS deletion prevented CMD along with the associated oxidative stress and diastolic and subclinical systolic dysfunction. The 1400W treatment reversed established CMD and oxidative stress and preserved systolic/diastolic function in mice fed a high-fat, high-sucrose diet. Thus, iNOS may represent a therapeutic target for CMD.
Keywords: HFpEF; cardiac MRI; coronary microvascular disease; iNOS.
© 2023 The Authors.
Conflict of interest statement
This study was supported by the National Institutes of Health National Institutes of Biomedical Imaging and Biomedical Engineering (R01 EB001763), Bethesda, Maryland; National Institutes of Health National Heart, Lung, and Blood Institute (R01 HL162872), Bethesda, Maryland; U.S.-Israel Binational Science Foundation grant BSF2017200, Jerusalem, Israel; and National Institute of General Medical Sciences Medical Scientist Training Program T32 grant T32GM007267, Bethesda, Maryland. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
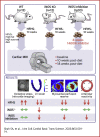

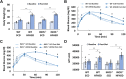



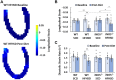
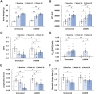

References
-
- Kaski J.-C., Crea F., Gersh B.J., Camici P.G. Reappraisal of ischemic heart disease: fundamental role of coronary microvascular dysfunction in the pathogenesis of angina pectoris. Circulation. 2018;138:1463–1480. - PubMed
-
- Yang J.H., Obokata M., Reddy Y.N.V., Redfield M.M., Lerman A., Borlaug B.A. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:432–441. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

