Calcification in Pulmonary Heart Valve Tissue Engineering: A Systematic Review and Meta-Analysis of Large-Animal Studies
- PMID: 37325410
- PMCID: PMC10264707
- DOI: 10.1016/j.jacbts.2022.09.009
Calcification in Pulmonary Heart Valve Tissue Engineering: A Systematic Review and Meta-Analysis of Large-Animal Studies
Abstract
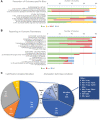
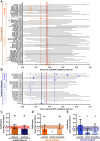
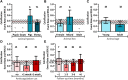
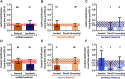
Tissue-engineered heart valves (TEHVs) are emerging alternatives to current valve prostheses and prospectively a lifelong replacement. Calcification, a pathological complication for biological protheses, has been reported in preclinical TEHV studies. Systematic analysis of its occurrence is missing. This review aims to: 1) systematically review reported calcification of pulmonary TEHVs in large-animal studies; and 2) analyze the influence of engineering methodology (choice of scaffold material, cell preseeding) and animal model (animal species and age) on calcification. Baseline analysis included 80 studies, of which 41 studies containing 108 experimental groups were included in meta-analysis. Inclusion was low because only 55% of studies reported on calcification. Meta-analysis showed an overall average calcification event rate of 35% (95% CI: 28%-43%). Calcification was more prominent (P = 0.023) in the arterial conduit region (34%; 95% CI: 26%-43%) than in the valve leaflets (21%; 95% CI: 17%-27%), and was mostly (42% in leaflets, 60% in conduits) present in a mild form. Time-analysis showed an initial surge within 1 month after implantation, decreased calcification between 1 and 3 months, and then progression over time. There were no significant differences in degree of calcification between TEHV strategy nor animal models. Much variability between individual studies was observed in degree of calcification as well as quality of analysis and reporting thereof, hampering adequate comparisons between studies. These findings underline the need for improved analysis and better reporting standards of calcification in TEHVs. It also necessitates control-based research to further enlighten the risk of calcification for tissue-engineered transplants compared to current options. This can bring the field of heart valve tissue engineering forward toward safe clinical use.
Keywords: biomaterials; cardiac valve prosthesis; mineralization; preclinical; regeneration; scaffold; tissue-engineered heart valve.
© 2023 The Authors.
Conflict of interest statement
This research was financially supported by the Gravitation Program “Materials Driven Regeneration,” funded by the Netherlands Organization for Scientific Research (024.003.013), and the More Knowledge with Fewer Animals program by ZonMw (Practice in Synthesis of Evidence grant 114024119). Dr Akiva has received support from the Netherlands Organization for Scientific Research through a VENI grant (VI.Veni.192.094). Dr Bouten is a shareholder of Xeltis BV. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




Similar articles
-
Pulmonary valve tissue engineering strategies in large animal models.PLoS One. 2021 Oct 5;16(10):e0258046. doi: 10.1371/journal.pone.0258046. eCollection 2021. PLoS One. 2021. PMID: 34610023 Free PMC article.
-
Transcatheter aortic valve implantation using anatomically oriented, marrow stromal cell-based, stented, tissue-engineered heart valves: technical considerations and implications for translational cell-based heart valve concepts.Eur J Cardiothorac Surg. 2014 Jan;45(1):61-8. doi: 10.1093/ejcts/ezt243. Epub 2013 May 8. Eur J Cardiothorac Surg. 2014. PMID: 23657551
-
Developing a Clinically Relevant Tissue Engineered Heart Valve-A Review of Current Approaches.Adv Healthc Mater. 2017 Dec;6(24). doi: 10.1002/adhm.201700918. Epub 2017 Nov 24. Adv Healthc Mater. 2017. PMID: 29171921 Review.
-
Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model.Sci Transl Med. 2018 May 9;10(440):eaan4587. doi: 10.1126/scitranslmed.aan4587. Sci Transl Med. 2018. PMID: 29743347
-
Trans-apical versus surgical implantation of autologous ovine tissue-engineered heart valves.J Heart Valve Dis. 2012 Sep;21(5):670-8. J Heart Valve Dis. 2012. PMID: 23167234
Cited by
-
A novel decellularization protocol with cryopreservation of pulmonary allografts in an ovine model.Interdiscip Cardiovasc Thorac Surg. 2025 Jun 4;40(6):ivaf131. doi: 10.1093/icvts/ivaf131. Interdiscip Cardiovasc Thorac Surg. 2025. PMID: 40465413 Free PMC article.
-
Performance of xenogeneic pulmonary visceral pleura as bioprosthetic heart valve cusps in swine.Front Cardiovasc Med. 2023 Aug 2;10:1213398. doi: 10.3389/fcvm.2023.1213398. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37600031 Free PMC article.
-
Biopolymer/Suture Polymer Interaction: Is It a Key of Bioprosthetic Calcification?Polymers (Basel). 2025 Jun 5;17(11):1576. doi: 10.3390/polym17111576. Polymers (Basel). 2025. PMID: 40508818 Free PMC article.
-
Reply: Evaluating Calcification in Tissue-Engineered Heart Valves: Much More Complicated Than Expected?JACC Basic Transl Sci. 2023 May 22;8(5):594-595. doi: 10.1016/j.jacbts.2023.04.002. eCollection 2023 May. JACC Basic Transl Sci. 2023. PMID: 37325401 Free PMC article. No abstract available.
-
In vitro model assesses the susceptibility of polymeric scaffolds for material-driven heart valve regeneration to calcification.In Vitro Model. 2025 Jul 15;4(2):157-175. doi: 10.1007/s44164-025-00090-x. eCollection 2025 Aug. In Vitro Model. 2025. PMID: 40708815 Free PMC article.
References
-
- Yacoub M.H., Takkenberg J.J.M. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med. 2005;2(2):60–61. - PubMed
-
- Glaser N., Persson M., Jackson V., Holzmann M.J., Franco-Cereceda A., Sartipy U. Loss in life expectancy after surgical aortic valve replacement. J Am Coll Cardiol. 2019;74(1):26–33. - PubMed
-
- Etnel J.R.G., Elmont L.C., Ertekin E., et al. Outcome after aortic valve replacement in children: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2016;151(1):143–152.e3. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

