zDHHC9 Regulates Cardiomyocyte Rab3a Activity and Atrial Natriuretic Peptide Secretion Through Palmitoylation of Rab3gap1
- PMID: 37325411
- PMCID: PMC10264568
- DOI: 10.1016/j.jacbts.2022.11.003
zDHHC9 Regulates Cardiomyocyte Rab3a Activity and Atrial Natriuretic Peptide Secretion Through Palmitoylation of Rab3gap1
Abstract
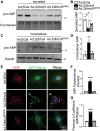
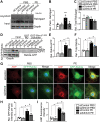
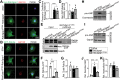
Production and release of natriuretic peptides by the stressed heart reduce cardiac workload by promoting vasodilation, natriuresis, and diuresis, which has been leveraged in the recent development of novel heart-failure pharmacotherapies, yet the mechanisms regulating cardiomyocyte exocytosis and natriuretic peptide release remain ill defined. We found that the Golgi S-acyltransferase zDHHC9 palmitoylates Rab3gap1 resulting in its spatial segregation from Rab3a, elevation of Rab3a-GTP levels, formation of Rab3a-positive peripheral vesicles, and impairment of exocytosis that limits atrial natriuretic peptide release. This novel pathway potentially can be exploited for targeting natriuretic peptide signaling in the treatment of heart failure.
Keywords: Rab3; atrial natriuretic peptide; exocytosis; heart failure; palmitoylation.
© 2023 The Authors.
Conflict of interest statement
This work was supported by grants from the National Institutes of Health (R00HL136695 to Dr Brody) and the American Heart Association (827440 to Dr Essandoh). All other authors have reported that they have no relationships relevant to the content of this manuscript to disclose.
Figures




References
-
- Schmidt M.F., Schlesinger M.J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979;17:813–819. - PubMed
-
- Prescott G.R., Gorleku O.A., Greaves J., Chamberlain L.H. Palmitoylation of the synaptic vesicle fusion machinery. J Neurochem. 2009;110:1135–1149. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

