Targeted and non-targeted proteomics to characterize the parasite proteins of Echinococcus multilocularis metacestodes
- PMID: 37325510
- PMCID: PMC10266102
- DOI: 10.3389/fcimb.2023.1170763
Targeted and non-targeted proteomics to characterize the parasite proteins of Echinococcus multilocularis metacestodes
Abstract
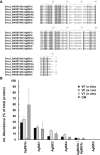
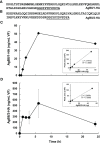
The larval stage of the cestode Echinococcus multilocularis is the causative agent of alveolar echinococcosis. To investigate the biology of these stages and to test novel compounds, metacestode cultures represent a suitable in vitro model system. These metacestodes are vesicles surrounded by an envelope formed by the vesicle tissue (VT), which is formed by the laminated and germinal layer, and filled with vesicle fluid (VF). We analyzed the proteome of VF and VT by liquid chromatography tandem mass spectrometry (LC-MS/MS) and identified a total of 2,954 parasite proteins. The most abundant protein in VT was the expressed conserved protein encoded by EmuJ_000412500, followed by the antigen B subunit AgB8/3a encoded by EmuJ_000381500 and Endophilin B1 (protein p29). In VF, the pattern was different and dominated by AgB subunits. The most abundant protein was the AgB8/3a subunit followed by three other AgB subunits. In total, the AgB subunits detected in VF represented 62.1% of the parasite proteins. In culture media (CM), 63 E. multilocularis proteins were detected, of which AgB subunits made up 93.7% of the detected parasite proteins. All AgB subunits detected in VF (encoded by EmuJ_000381100-700, corresponding to AgB8/2, AgB8/1, AgB8/4, AgB8/3a, AgB8/3b, and AgB8/3c) were also found in CM, except the subunit encoded by EmuJ_000381800 (AgB8/5) that was very rare in VF and not detected in CM. The relative abundance of the AgB subunits in VF and CM followed the same pattern. In VT, only the subunits EmuJ_000381500 (AgB8/3a) and EmuJ_000381200 (AgB8/1) were detected among the 20 most abundant proteins. To see whether this pattern was specific to VF from in vitro cultured metacestodes, we analyzed the proteome of VF from metacestodes grown in a mouse model. Here, the AgB subunits encoded by EmuJ_000381100-700 constituted the most abundant proteins, namely, 81.9% of total protein, with the same order of abundance as in vitro. Immunofluorescence on metacestodes showed that AgB is co-localized to calcareous corpuscles of E. multilocularis. Using targeted proteomics with HA-tagged EmuJ_000381200 (AgB8/1) and EmuJ_000381100 (AgB8/2), we could show that uptake of AgB subunits from CM into VF occurs within hours.
Keywords: cestodes; antigen B; echinococcosis; model system; targeted proteomics; transport; untargeted proteomics.
Copyright © 2023 Müller, Preza, Kaethner, Rufener, Braga, Uldry, Heller and Lundström-Stadelmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Characterisation of Antigen B Protein Species Present in the Hydatid Cyst Fluid of Echinococcus canadensis G7 Genotype.PLoS Negl Trop Dis. 2017 Jan 3;11(1):e0005250. doi: 10.1371/journal.pntd.0005250. eCollection 2017 Jan. PLoS Negl Trop Dis. 2017. PMID: 28045899 Free PMC article.
-
Echinococcus granulosus antigen B structure: subunit composition and oligomeric states.PLoS Negl Trop Dis. 2012;6(3):e1551. doi: 10.1371/journal.pntd.0001551. Epub 2012 Mar 6. PLoS Negl Trop Dis. 2012. PMID: 22413028 Free PMC article.
-
In vitro metabolomic footprint of the Echinococcus multilocularis metacestode.Sci Rep. 2019 Dec 19;9(1):19438. doi: 10.1038/s41598-019-56073-y. Sci Rep. 2019. PMID: 31857639 Free PMC article.
-
Recombinant subunits as tools for the structural and functional characterization of Echinococcus granulosus antigen B.Exp Parasitol. 2008 Aug;119(4):490-498. doi: 10.1016/j.exppara.2008.04.015. Epub 2008 Apr 24. Exp Parasitol. 2008. PMID: 18513717 Review.
-
In vitro culture of Echinococcus multilocularis and Echinococcus vogeli metacestodes: studies on the host-parasite interface.Acta Trop. 2003 Feb;85(2):145-55. doi: 10.1016/s0001-706x(02)00220-6. Acta Trop. 2003. PMID: 12606091 Review.
Cited by
-
Identification and characterization of the elusive protein backbone of the immuno-dominant and species-specific Em2(G11) metacestode antigen of Echinococcus multilocularis.Front Parasitol. 2025 Mar 11;4:1540215. doi: 10.3389/fpara.2025.1540215. eCollection 2025. Front Parasitol. 2025. PMID: 40135073 Free PMC article.
-
Human Alveolar Echinococcosis-A Neglected Zoonotic Disease Requiring Urgent Attention.Int J Mol Sci. 2025 Mar 19;26(6):2784. doi: 10.3390/ijms26062784. Int J Mol Sci. 2025. PMID: 40141427 Free PMC article. Review.
-
Host Proteins in Echinococcus multilocularis Metacestodes.Int J Mol Sci. 2025 Apr 1;26(7):3266. doi: 10.3390/ijms26073266. Int J Mol Sci. 2025. PMID: 40244114 Free PMC article.
-
Preliminary Proteomic and Metabolomic Analyses Reveal Potential Serum Biomarkers for Identifying Alveolar Echinococcosis in Mice.Vet Sci. 2025 Jun 9;12(6):565. doi: 10.3390/vetsci12060565. Vet Sci. 2025. PMID: 40559802 Free PMC article.
-
Investigation of the threonine metabolism of Echinococcus multilocularis: The threonine dehydrogenase as a potential drug target in alveolar echinococcosis.Int J Parasitol Drugs Drug Resist. 2025 Apr;27:100581. doi: 10.1016/j.ijpddr.2025.100581. Epub 2025 Jan 18. Int J Parasitol Drugs Drug Resist. 2025. PMID: 39847910 Free PMC article.
References
-
- Ahn C. S., Kim J. G., Han X., Bae Y. A., Park W. J., Kang I., et al. . (2017. b). Biochemical characterization of Echinococcus multilocularis antigen B3 reveals insight into adaptation and maintenance of parasitic homeostasis at the host-parasite interface. J. Proteome Res. 16, 806–823. doi: 10.1021/acs.jproteome.6b00799 - DOI - PubMed
-
- Ahn C. S., Kim J. G., Han X., Kang I., Kong Y. (2017. c). Comparison of Echinococcus multilocularis and Echinococcus granulosus hydatid fluid proteome provides molecular strategies for specialized host-parasite interactions. Oncotarget 8, 97009–97024. doi: 10.18632/oncotarget.20761 - DOI - PMC - PubMed

