Oncolytic herpes simplex viruses for the treatment of glioma and targeting glioblastoma stem-like cells
- PMID: 37325516
- PMCID: PMC10264819
- DOI: 10.3389/fcimb.2023.1206111
Oncolytic herpes simplex viruses for the treatment of glioma and targeting glioblastoma stem-like cells
Abstract
Glioblastoma (GBM) is one of the most lethal cancers, having a poor prognosis and a median survival of only about 15 months with standard treatment (surgery, radiation, and chemotherapy), which has not been significantly extended in decades. GBM demonstrates remarkable cellular heterogeneity, with glioblastoma stem-like cells (GSCs) at the apex. GSCs are a subpopulation of GBM cells that possess the ability to self-renew, differentiate, initiate tumor formation, and manipulate the tumor microenvironment (TME). GSCs are no longer considered a static population of cells with specific markers but are quite flexible phenotypically and in driving tumor heterogeneity and therapeutic resistance. In light of these features, they are a critical target for successful GBM therapy. Oncolytic viruses, in particular oncolytic herpes simplex viruses (oHSVs), have many attributes for therapy and are promising agents to target GSCs. oHSVs are genetically-engineered to selectively replicate in and kill cancer cells, including GSCs, but not normal cells. Moreover, oHSV can induce anti-tumor immune responses and synergize with other therapies, such as chemotherapy, DNA repair inhibitors, and immune checkpoint inhibitors, to potentiate treatment effects and reduce GSC populations that are partly responsible for chemo- and radio-resistance. Herein, we present an overview of GSCs, activity of different oHSVs, clinical trial results, and combination strategies to enhance efficacy, including therapeutic arming of oHSV. Throughout, the therapeutic focus will be on GSCs and studies specifically targeting these cells. Recent clinical trials and approval of oHSV G47Δ in Japan for patients with recurrent glioma demonstrate the efficacy and promise of oHSV therapy.
Keywords: GSC; cancer stem cell; glioblastoma; immunotherapy; oHSV; oncolytic virus; virotherapy.
Copyright © 2023 Kardani, Sanchez Gil and Rabkin.
Conflict of interest statement
Author SR is a co-inventor on patents relating to oncolytic herpes simplex viruses, owned and managed by Georgetown University and Massachusetts General Hospital, which have received royalties from Amgen and Acti\Vec Inc, and acted as a consultant and received honoraria from Replimune, Cellinta, and Greenfire Bio, and honoraria and equity from EG 427. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
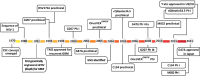
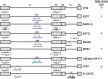
 ).
).Similar articles
-
Restriction of Replication of Oncolytic Herpes Simplex Virus with a Deletion of γ34.5 in Glioblastoma Stem-Like Cells.J Virol. 2018 Jul 17;92(15):e00246-18. doi: 10.1128/JVI.00246-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29793956 Free PMC article.
-
Blockade of transforming growth factor-β signaling enhances oncolytic herpes simplex virus efficacy in patient-derived recurrent glioblastoma models.Int J Cancer. 2017 Dec 1;141(11):2348-2358. doi: 10.1002/ijc.30929. Epub 2017 Aug 26. Int J Cancer. 2017. PMID: 28801914 Free PMC article.
-
Oncolytic herpes simplex virus counteracts the hypoxia-induced modulation of glioblastoma stem-like cells.Stem Cells Transl Med. 2012 Apr;1(4):322-32. doi: 10.5966/sctm.2011-0035. Epub 2012 Mar 21. Stem Cells Transl Med. 2012. PMID: 23197811 Free PMC article.
-
Implications of immune cells in oncolytic herpes simplex virotherapy for glioma.Brain Tumor Pathol. 2022 Apr;39(2):57-64. doi: 10.1007/s10014-022-00431-8. Epub 2022 Apr 6. Brain Tumor Pathol. 2022. PMID: 35384530 Review.
-
Oncolytic virotherapy improves immunotherapies targeting cancer stemness in glioblastoma.Biochim Biophys Acta Gen Subj. 2024 Sep;1868(9):130662. doi: 10.1016/j.bbagen.2024.130662. Epub 2024 Jun 18. Biochim Biophys Acta Gen Subj. 2024. PMID: 38901497 Review.
Cited by
-
The potential of gene delivery for the treatment of traumatic brain injury.J Neuroinflammation. 2024 Jul 28;21(1):183. doi: 10.1186/s12974-024-03156-x. J Neuroinflammation. 2024. PMID: 39069631 Free PMC article. Review.
-
Mathematical modelling of reoviruses in cancer cell cultures.PLoS One. 2025 Apr 28;20(4):e0318078. doi: 10.1371/journal.pone.0318078. eCollection 2025. PLoS One. 2025. PMID: 40294035 Free PMC article.
-
Repurposing of Zika virus live-attenuated vaccine (ZIKV-LAV) strains as oncolytic viruses targeting human glioblastoma multiforme cells.J Transl Med. 2024 Feb 2;22(1):126. doi: 10.1186/s12967-024-04930-4. J Transl Med. 2024. PMID: 38308299 Free PMC article.
-
Harnessing immunotherapy: cancer vaccines as novel therapeutic strategies for brain tumor.Front Immunol. 2025 Jul 17;16:1588081. doi: 10.3389/fimmu.2025.1588081. eCollection 2025. Front Immunol. 2025. PMID: 40746549 Free PMC article. Review.
-
Oncolytic Therapies for Glioblastoma: Advances, Challenges, and Future Perspectives.Cancers (Basel). 2025 Aug 1;17(15):2550. doi: 10.3390/cancers17152550. Cancers (Basel). 2025. PMID: 40805247 Free PMC article. Review.
References
-
- Advani S. J., Markert J. M., Sood R. F., Samuel S., Gillespie G. Y., Shao M. Y., et al. . (2011). Increased oncolytic efficacy for high-grade gliomas by optimal integration of ionizing radiation into the replicative cycle of HSV-1. Gene Ther. 18 (11), 1098–1102. doi: 10.1038/gt.2011.61 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

