Accounts of applied molecular rotors and rotary motors: recent advances
- PMID: 37325522
- PMCID: PMC10262963
- DOI: 10.1039/d3na00010a
Accounts of applied molecular rotors and rotary motors: recent advances
Abstract
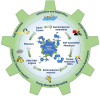
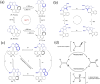
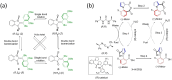
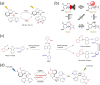
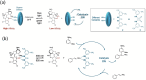
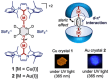
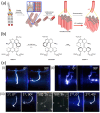
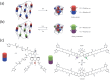
Molecular machines are nanoscale devices capable of performing mechanical works at molecular level. These systems could be a single molecule or a collection of component molecules that interrelate with one another to produce nanomechanical movements and resulting performances. The design of the components of molecular machine with bioinspired traits results in various nanomechanical motions. Some known molecular machines are rotors, motors, nanocars, gears, elevators, and so on based on their nanomechanical motion. The conversion of these individual nanomechanical motions to collective motions via integration into suitable platforms yields impressive macroscopic output at varied sizes. Instead of limited experimental acquaintances, the researchers demonstrated several applications of molecular machines in chemical transformation, energy conversion, gas/liquid separation, biomedical use, and soft material fabrication. As a result, the development of new molecular machines and their applications has accelerated over the previous two decades. This review highlights the design principles and application scopes of several rotors and rotary motor systems because these machines are used in real applications. This review also offers a systematic and thorough overview of current advancements in rotary motors, providing in-depth knowledge and predicting future problems and goals in this area.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare there are no conflicts of interest.
Figures













Similar articles
-
Design of Collective Motions from Synthetic Molecular Switches, Rotors, and Motors.Chem Rev. 2020 Jan 8;120(1):310-433. doi: 10.1021/acs.chemrev.9b00288. Epub 2019 Dec 23. Chem Rev. 2020. PMID: 31869214
-
Coupled Rotary Motion in Molecular Motors.J Am Chem Soc. 2024 Feb 28;146(8):5634-5642. doi: 10.1021/jacs.3c14430. Epub 2024 Feb 13. J Am Chem Soc. 2024. PMID: 38350104 Free PMC article.
-
Making molecular machines work.Nat Nanotechnol. 2006 Oct;1(1):25-35. doi: 10.1038/nnano.2006.45. Nat Nanotechnol. 2006. PMID: 18654138 Review.
-
Designing light-driven rotary molecular motors.Chem Sci. 2021 Oct 20;12(45):14964-14986. doi: 10.1039/d1sc04781g. eCollection 2021 Nov 24. Chem Sci. 2021. PMID: 34909140 Free PMC article. Review.
-
Photo-responsive Helical Motion by Light-Driven Molecular Motors in a Liquid-Crystal Network.Angew Chem Int Ed Engl. 2021 Apr 6;60(15):8251-8257. doi: 10.1002/anie.202016254. Epub 2021 Mar 12. Angew Chem Int Ed Engl. 2021. PMID: 33511680 Free PMC article.
Cited by
-
A Cost-Effective Computational Strategy for the Electronic Layout Characterization of a Second Generation Light-Driven Molecular Rotary Motor in Solution.J Comput Chem. 2025 Jan 15;46(2):e70023. doi: 10.1002/jcc.70023. J Comput Chem. 2025. PMID: 39797623 Free PMC article.
-
Stabilization of Hoogsteen H-bonds in G-quartet sheets by coordinated K+ ion for enhanced efficiency in guanine-rich DNA nanomotor.Bioimpacts. 2025 May 3;15:30596. doi: 10.34172/bi.30596. eCollection 2025. Bioimpacts. 2025. PMID: 40584909 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

