How to train your myeloid cells: a way forward for helminth vaccines?
- PMID: 37325618
- PMCID: PMC10266106
- DOI: 10.3389/fimmu.2023.1163364
How to train your myeloid cells: a way forward for helminth vaccines?
Abstract
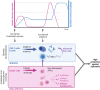
Soil-transmitted helminths affect approximately 1.5 billion people worldwide. However, as no vaccine is currently available for humans, the current strategy for elimination as a public health problem relies on preventive chemotherapy. Despite more than 20 years of intense research effort, the development of human helminth vaccines (HHVs) has not yet come to fruition. Current vaccine development focuses on peptide antigens that trigger strong humoral immunity, with the goal of generating neutralizing antibodies against key parasite molecules. Notably, this approach aims to reduce the pathology of infection, not worm burden, with only partial protection observed in laboratory models. In addition to the typical translational hurdles that vaccines struggle to overcome, HHVs face several challenges (1): helminth infections have been associated with poor vaccine responses in endemic countries, probably due to the strong immunomodulation caused by these parasites, and (2) the target population displays pre-existing type 2 immune responses to helminth products, increasing the likelihood of adverse events such as allergy or anaphylaxis. We argue that such traditional vaccines are unlikely to be successful on their own and that, based on laboratory models, mucosal and cellular-based vaccines could be a way to move forward in the fight against helminth infection. Here, we review the evidence for the role of innate immune cells, specifically the myeloid compartment, in controlling helminth infections. We explore how the parasite may reprogram myeloid cells to avoid killing, notably using excretory/secretory (ES) proteins and extracellular vesicles (EVs). Finally, learning from the field of tuberculosis, we will discuss how anti-helminth innate memory could be harnessed in a mucosal-trained immunity-based vaccine.
Keywords: cellular immunity; helminth; myeloid cells; trained immunity; vaccine.
Copyright © 2023 Doolan, Putananickal, Tritten and Bouchery.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Chronic helminth infections induce immunomodulation: consequences and mechanisms.Immunobiology. 2007;212(6):475-90. doi: 10.1016/j.imbio.2007.03.009. Epub 2007 Apr 20. Immunobiology. 2007. PMID: 17544832 Review.
-
Extracellular vesicles: new targets for vaccines against helminth parasites.Int J Parasitol. 2020 Aug;50(9):623-633. doi: 10.1016/j.ijpara.2020.04.011. Epub 2020 Jul 11. Int J Parasitol. 2020. PMID: 32659278 Free PMC article. Review.
-
Pre-existing helminth infection impairs the efficacy of adjuvanted influenza vaccination in mice.PLoS One. 2022 Mar 31;17(3):e0266456. doi: 10.1371/journal.pone.0266456. eCollection 2022. PLoS One. 2022. PMID: 35358281 Free PMC article.
-
Vaccination against helminth parasite infections.Expert Rev Vaccines. 2014 Apr;13(4):473-87. doi: 10.1586/14760584.2014.893195. Expert Rev Vaccines. 2014. PMID: 24606541 Review.
-
What Can Parasites Tell Us About the Pathogenesis and Treatment of Asthma and Allergic Diseases.Front Immunol. 2020 Sep 11;11:2106. doi: 10.3389/fimmu.2020.02106. eCollection 2020. Front Immunol. 2020. PMID: 33013887 Free PMC article. Review.
Cited by
-
Trained immunity: a cutting edge approach for designing novel vaccines against parasitic diseases?Front Immunol. 2023 Oct 6;14:1252554. doi: 10.3389/fimmu.2023.1252554. eCollection 2023. Front Immunol. 2023. PMID: 37868995 Free PMC article. Review.
-
Anti-Inflammatory Effects of Helminth-Derived Products: Potential Applications and Challenges in Diabetes Mellitus Management.J Inflamm Res. 2024 Dec 28;17:11789-11812. doi: 10.2147/JIR.S493374. eCollection 2024. J Inflamm Res. 2024. PMID: 39749005 Free PMC article. Review.
-
Proteomic characterization and comparison of the infective and adult life stage secretomes from Necator americanus and Ancylostoma ceylanicum.PLoS Negl Trop Dis. 2025 Jan 20;19(1):e0012780. doi: 10.1371/journal.pntd.0012780. eCollection 2025 Jan. PLoS Negl Trop Dis. 2025. PMID: 39832284 Free PMC article.
References
-
- Avramenko RW, Redman EM, Windeyer C, Gilleard JS. Assessing anthelmintic resistance risk in the post-genomic era: a proof-of-concept study assessing the potential for widespread benzimidazole-resistant gastrointestinal nematodes in north American cattle and bison. Parasitology (2020) 147(8):897–906. doi: 10.1017/S0031182020000426 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

