The multikinase inhibitor axitinib in the treatment of advanced hepatocellular carcinoma: the current clinical applications and the molecular mechanisms
- PMID: 37325670
- PMCID: PMC10264605
- DOI: 10.3389/fimmu.2023.1163967
The multikinase inhibitor axitinib in the treatment of advanced hepatocellular carcinoma: the current clinical applications and the molecular mechanisms
Abstract
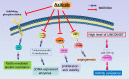
Advanced hepatocellular carcinoma (HCC) is a formidable public health problem with limited curable treatment options. Axitinib, an oral tyrosine kinase inhibitor, is a potent and selective second-generation inhibitor of vascular endothelial growth factor receptor (VEGFR) 1, 2, and 3. This anti-angiogenic drug was found to have promising activity in various solid tumors, including advanced HCC. At present, however, there is no relevant review article that summarizes the exact roles of axitinib in advanced HCC. In this review, 24 eligible studies (seven studies in the ClinicalTrials, eight experimental studies, and nine clinical trials) were included for further evaluation. The included randomized or single-arm phase II trials indicated that axitinib could not prolong the overall survival compared to the placebo for the treatment of advanced HCC, but improvements in progression free survival and time to tumor progression were observed. Experimental studies showed that the biochemical effects of axitinib in HCC might be regulated by its associated genes and affected signaling cascades (e.g. VEGFR2/PAK1, CYP1A2, CaMKII/ERK, Akt/mTor, and miR-509-3p/PDGFRA). FDA approved sorafenib combined with nivolumab (an inhibitor of PD-1/PD-L1) as the first line regimen for the treatment of advanced HCC. Since both axitinib and sorafenib are tyrosine kinase inhibitors as well as the VEGFR inhibitors, axitinib combined with anti-PDL-1/PD-1 antibodies may also exhibit tremendous potential in anti-tumoral effects for advanced HCC. The present review highlights the current clinical applications and the molecular mechanisms of axitinib in advanced HCC. To move toward clinical applications by combining axitinib and other treatments in advanced HCC, more studies are still warranted in the near future.
Keywords: axitinib; hepatocellular carcinoma; mechanism; survival; tyrosine kinase.
Copyright © 2023 Jiang, Liao, Wang, Jin, Mo and Xiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A phase II trial of second-line axitinib following prior antiangiogenic therapy in advanced hepatocellular carcinoma.Cancer. 2015 May 15;121(10):1620-7. doi: 10.1002/cncr.29227. Epub 2015 Jan 6. Cancer. 2015. PMID: 25565269 Clinical Trial.
-
A Multicenter Phase II Study of Second-Line Axitinib for Patients with Advanced Hepatocellular Carcinoma Failing First-Line Sorafenib Monotherapy.Oncologist. 2020 Sep;25(9):e1280-e1285. doi: 10.1634/theoncologist.2020-0143. Epub 2020 Apr 9. Oncologist. 2020. PMID: 32271494 Free PMC article. Clinical Trial.
-
Randomized phase II study of axitinib versus placebo plus best supportive care in second-line treatment of advanced hepatocellular carcinoma.Ann Oncol. 2015 Dec;26(12):2457-63. doi: 10.1093/annonc/mdv388. Epub 2015 Sep 18. Ann Oncol. 2015. PMID: 26386123 Clinical Trial.
-
Drug Treatment for Advanced Hepatocellular Carcinoma: First-Line and Beyond.Curr Oncol. 2022 Aug 4;29(8):5489-5507. doi: 10.3390/curroncol29080434. Curr Oncol. 2022. PMID: 36005172 Free PMC article. Review.
-
[Clinical research progression of molecular-targeted drugs and PD-1 inhibitors for advanced hepatocellular carcinoma].Zhonghua Zhong Liu Za Zhi. 2019 Jun 23;41(6):406-409. doi: 10.3760/cma.j.issn.0253-3766.2019.06.002. Zhonghua Zhong Liu Za Zhi. 2019. PMID: 31216824 Review. Chinese.
Cited by
-
CRISPR/Cas-Mediated Knockdown of PD-L1 and KRAS in Lung Cancer Cells.Int J Mol Sci. 2024 Aug 22;25(16):9086. doi: 10.3390/ijms25169086. Int J Mol Sci. 2024. PMID: 39201772 Free PMC article.
-
Small molecule tyrosine kinase inhibitors approved for systemic therapy of advanced hepatocellular carcinoma: recent advances and future perspectives.Discov Oncol. 2024 Jul 3;15(1):259. doi: 10.1007/s12672-024-01110-0. Discov Oncol. 2024. PMID: 38960980 Free PMC article. Review.
-
Construction of an endoplasmic reticulum stress and cuproptosis -related lncRNAs signature in chemosensitivity in hepatocellular carcinoma by comprehensive bioinformatics analysis.Heliyon. 2024 Sep 24;10(19):e38342. doi: 10.1016/j.heliyon.2024.e38342. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39398070 Free PMC article.
-
LINC01614 is a promising diagnostic and prognostic marker in HNSC linked to the tumor microenvironment and oncogenic function.Front Genet. 2024 Apr 9;15:1337525. doi: 10.3389/fgene.2024.1337525. eCollection 2024. Front Genet. 2024. PMID: 38655053 Free PMC article.
-
Cardiovascular toxicities associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a pharmacovigilance study based on FDA adverse event reporting system.Int J Clin Pharm. 2025 Jul 2. doi: 10.1007/s11096-025-01962-8. Online ahead of print. Int J Clin Pharm. 2025. PMID: 40601086
References
-
- Nischalke HD, Berger C, Luda C, Berg T, Muller T, Grunhage F, et al. . The PNPLA3 rs738409 148M/M genotype is a risk factor for liver cancer in alcoholic cirrhosis but shows no or weak association in hepatitis c cirrhosis. PloS One (2011) 6:e27087. doi: 10.1371/journal.pone.0027087 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous