PDGF-BB-Dependent Neurogenesis Buffers Depressive-Like Behaviors by Inhibition of GABAergic Projection from Medial Septum to Dentate Gyrus
- PMID: 37325895
- PMCID: PMC10401107
- DOI: 10.1002/advs.202301110
PDGF-BB-Dependent Neurogenesis Buffers Depressive-Like Behaviors by Inhibition of GABAergic Projection from Medial Septum to Dentate Gyrus
Abstract
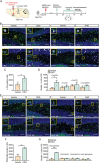
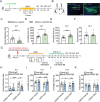
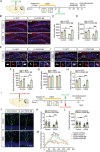
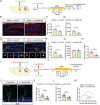
Hippocampal circuitry stimulation is sufficient to regulate adult hippocampal neurogenesis and ameliorate depressive-like behavior, but its underlying mechanism remains unclear. Here, it is shown that inhibition of medial septum (MS)-dentate gyrus (DG) circuit reverses the chronic social defeat stress (CSDS)-induced depression-like behavior. Further analysis exhibits that inhibition of gamma-aminobutyric acidergic neurons in MS projecting to the DG (MSGABA+ -DG) increases the expression of platelet-derived growth factor-BB (PDGF-BB) in somatostatin (SOM) positive interneurons of DG, which contributes to the antidepressant-like effects. Overexpression of the PDGF-BB or exogenous administration of PDGF-BB in DG rescues the effect of chronic stress on the inhibition of neural stem cells (NSCs) proliferation and dendritic growth of adult-born hippocampal neurons, as well as on depressive-like behaviors. Conversely, knockdown of PDGF-BB facilitates CSDS-induced deficit of hippocampal neurogenesis and promotes the susceptibility to chronic stress in mice. Finally, conditional knockdown platelet-derived growth factor receptor beta (PDGFRβ) in NSCs blocks an increase in NSCs proliferation and the antidepressant effects of PDGF-BB. These results delineate a previously unidentified PDGF-BB/PDGFRβ signaling in regulating depressive-like behaviors and identify a novel mechanism by which the MSGABA+ -DG pathway regulates the expression of PDGF-BB in SOM-positive interneurons.
Keywords: MS-DG pathway; PDGF-BB; depressive-like behaviors; neurogenesis.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Leuner B., Gould E., Annu. Rev. Psychol. 2010, 61, 111; - PMC - PubMed
- b) Yun S., Reynolds R. P., Masiulis I., Eisch A. J., Nat. Med. 2016, 22, 1239; - PMC - PubMed
- c) Boldrini M., Santiago A. N., Hen R., Dwork A. J., Rosoklija G. B., Tamir H., Arango V., Mann J. John, Neuropsychopharmacology 2013, 38, 1068; - PMC - PubMed
- d) Milne A., MacQueen G. M., Yucel K., Soreni N., Hall G. B., NeuroImage 2009, 47, 36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
