Optimizing the Design of Clinical Trials to Evaluate the Efficacy of Function-Promoting Therapies
- PMID: 37325959
- PMCID: PMC10272979
- DOI: 10.1093/gerona/glad024
Optimizing the Design of Clinical Trials to Evaluate the Efficacy of Function-Promoting Therapies
Abstract
Background: Several candidate molecules that may have application in treating physical limitations associated with aging and chronic diseases are in development. Challenges in the framing of indications, eligibility criteria, and endpoints and the lack of regulatory guidance have hindered the development of function-promoting therapies.
Methods: Experts from academia, pharmaceutical industry, National Institutes of Health (NIH), and Food and Drug Administration (FDA) discussed optimization of trial design including the framing of indications, eligibility criteria, and endpoints.
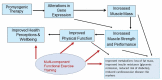
Results: Mobility disability associated with aging and chronic diseases is an attractive indication because it is recognized by geriatricians as a common condition associated with adverse outcomes, and it can be ascertained reliably. Other conditions associated with functional limitation in older adults include hospitalization for acute illnesses, cancer cachexia, and fall injuries. Efforts are underway to harmonize definitions of sarcopenia and frailty. Eligibility criteria should reconcile the goals of selecting participants with the condition and ensuring generalizability and ease of recruitment. An accurate measure of muscle mass (eg, D3 creatine dilution) could be a good biomarker in early-phase trials. Performance-based and patient-reported measures of physical function are needed to demonstrate whether treatment improves how a person lives, functions, or feels. Multicomponent functional training that integrates training in balance, stability, strength, and functional tasks with cognitive and behavioral strategies may be needed to translate drug-induced muscle mass gains into functional improvements.
Conclusions: Collaborations among academic investigators, NIH, FDA, pharmaceutical industry, patients, and professional societies are needed to conduct well-designed trials of function-promoting pharmacological agents with and without multicomponent functional training.
Keywords: Clinical trial design; Function-promoting drugs; Functional decline with aging; Sarcopenia; Skeletal muscle dysfunction.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
S.B. reports receiving research grants from National Institute on Aging (NIA), National Institute of Nursing Research, National Institute of Child Health and Human Development-National Center for Medical Rehabilitation Research, AbbVie, Metro International Biotechnology, and Function Promoting Therapies, LLC (FPT) that are managed by the Brigham and Women’s Hospital; consultation fees from Aditum and Novartis, and equity interest in FPT and Xyone. These conflicts are overseen and managed in accordance with the rules and policies of the MGB Office of Industry Interaction. E.V. is supported in part by the UTMB Claude D. Pepper Older Americans Independence Center P30 AG024832. E.V. received grant funding as principal investigator from the National Institutes of Health (NIH) and the Dairy Research Institute. R.A.F. is partially supported by the U.S. Department of Agriculture (USDA), under agreement No. 58-8050-9-004 and by Boston Claude D. Pepper Center (OAIC; 1P30AG031679). Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. R.A.F. reports consulting not related to the present work from: Pfizer, Biophytis, Amazentis, Rejuventate Biomed, and Nestle; and grant support from Lonza and Biophytis. Any opinions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the NIH, including the NIA and National Institute of Arthritis, Musculoskeletal and Skin Diseases; the U.S. Food and Drug Administration (FDA), or the U.S. Department of Health and Human Services.
Figures