Treatment outcome comparisons of first-line targeted therapy in patients with KRAS wild-type metastatic colorectal cancer: A nationwide database study
- PMID: 37325970
- PMCID: PMC10417087
- DOI: 10.1002/cam4.6196
Treatment outcome comparisons of first-line targeted therapy in patients with KRAS wild-type metastatic colorectal cancer: A nationwide database study
Abstract
Background: The first-line systemic therapy for metastatic colorectal cancer (mCRC) is a combination of one targeted therapy agent and a chemotherapy doublet. Whether bevacizumab or anti-epidermal growth factor receptor (anti-EGFR) monoclonal antibody (mAb) is the more effective addition to a chemotherapy doublet as the first-line treatment for inoperable KRAS wild-type mCRC remains controversial in prior clinical trials. Moreover, the association between the sidedness of primary tumors and the efficacy of anti-EGFR mAb needs to be addressed.
Methods: We established a cohort of patients with KRAS wild-type mCRC who were treated with first-line targeted therapy plus doublet chemotherapy between 2013 and 2018 using Taiwan's National Health Insurance Research Database. Secondary surgery was defined as either resection of primary tumors, liver metastases, lung metastases, or radiofrequency ablation.
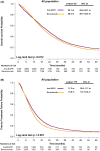
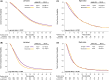
Results: A total of 6482 patients were included; bevacizumab and anti-EGFR mAb were the first-line targeted therapies in 3334 (51.4%) and 3148 (48.6%) patients, respectively. Compared with those who received bevacizumab, patients who received anti-EGFR mAb exhibited significantly longer overall survival (OS; median, 23.1 vs. 20.2 months, p = 0.012) and time to treatment failure (TTF; median, 11.3 vs. 10 months, p < 0.001). Among left-sided primary tumors, the OS and TTF benefits of anti-EGFR mAb remained. Among right-sided primary tumors, the OS and TTF were similar regardless of the type of targeted therapy. In multivariate analyses, first-line anti-EGFR mAb therapy remained an independent predictor of longer OS and TTF for left-sided primary tumors. Patients who received anti-EGFR mAb were more likely to receive secondary surgery (29.6% vs. 22.6%, p < 0.0001) than patients who received bevacizumab.
Conclusion: For patients who received first-line doublet chemotherapy for KRAS wild-type mCRC, adding anti-EGFR mAb was associated with significantly longer OS and TTF, especially for left-sided primary tumors.
Keywords: EGFR; bevacizumab; cetuximab; metastatic colorectal cancer; panitumumab; secondary surgery.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors claim that there is no any conflict of interest.
Figures

Similar articles
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
-
Panitumumab vs Bevacizumab Added to Standard First-line Chemotherapy and Overall Survival Among Patients With RAS Wild-type, Left-Sided Metastatic Colorectal Cancer: A Randomized Clinical Trial.JAMA. 2023 Apr 18;329(15):1271-1282. doi: 10.1001/jama.2023.4428. JAMA. 2023. PMID: 37071094 Free PMC article. Clinical Trial.
-
Primary tumour side as a driver for treatment choice in RAS wild-type metastatic colorectal cancer patients: a systematic review and pooled analysis of randomised trials.Eur J Cancer. 2023 May;184:106-116. doi: 10.1016/j.ejca.2023.02.006. Epub 2023 Feb 17. Eur J Cancer. 2023. PMID: 36913832
-
Fluoropyrimidine type, patient age, tumour sidedness and mutation status as determinants of benefit in patients with metastatic colorectal cancer treated with EGFR monoclonal antibodies: individual patient data pooled analysis of randomised trials from the ARCAD database.Br J Cancer. 2024 May;130(8):1269-1278. doi: 10.1038/s41416-024-02604-y. Epub 2024 Feb 24. Br J Cancer. 2024. PMID: 38402342 Free PMC article. Clinical Trial.
-
Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review.Clin Ther. 2010 Mar;32(3):437-53. doi: 10.1016/j.clinthera.2010.03.012. Clin Ther. 2010. PMID: 20399983 Review.
Cited by
-
Continuing anti-EGFR monoclonal antibody after secondary resection significantly prolongs overall survival for patients with metastatic colorectal cancer who were responsive to first-line anti-EGFR monoclonal antibody plus chemotherapy doublet.Am J Cancer Res. 2024 Dec 15;14(12):5909-5920. doi: 10.62347/MUCQ4129. eCollection 2024. Am J Cancer Res. 2024. PMID: 39803663 Free PMC article.
-
Final Results of ERBIMOX: A Randomized Phase II Study of Modified FOLFOX7 With or Without Cetuximab as First-Line Treatment for KRAS Wild-type Metastatic Colorectal Cancer.J Gastrointest Cancer. 2025 Jun 27;56(1):141. doi: 10.1007/s12029-025-01260-6. J Gastrointest Cancer. 2025. PMID: 40571867 Clinical Trial.
References
-
- Kao CW, Chiang CJ, Lin LJ, Huang CW, Lee WC, Lee MY. Accuracy of long‐form data in the Taiwan cancer registry. J Formos Med Assoc. 2021;120:2037‐2041. - PubMed
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. - PubMed
-
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386‐1422. - PubMed
-
- Yoshino T, Arnold D, Taniguchi H, et al. Pan‐Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO‐ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29:44‐70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

