Uncertainty management in regulatory and health technology assessment decision-making on drugs: guidance of the HTAi-DIA Working Group
- PMID: 37325997
- PMCID: PMC11570246
- DOI: 10.1017/S0266462323000375
Uncertainty management in regulatory and health technology assessment decision-making on drugs: guidance of the HTAi-DIA Working Group
Abstract
Objectives: Uncertainty is a fundamental component of decision making regarding access to and pricing and reimbursement of drugs. The context-specific interpretation and mitigation of uncertainty remain major challenges for decision makers. Following the 2021 HTAi Global Policy Forum, a cross-sectoral, interdisciplinary HTAi-DIA Working Group (WG) was initiated to develop guidance to support stakeholder deliberation on the systematic identification and mitigation of uncertainties in the regulatory-HTA interface.
Methods: Six online discussions among WG members (Dec 2021-Sep 2022) who examined the output of a scoping review, two literature-based case studies and a survey; application of the initial guidance to a real-world case study; and two international conference panel discussions.
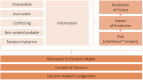
Results: The WG identified key concepts, clustered into twelve building blocks that were collectively perceived to define uncertainty: "unavailable," "inaccurate," "conflicting," "not understandable," "random variation," "information," "prediction," "impact," "risk," "relevance," "context," and "judgment." These were converted into a checklist to explain and define whether any issue constitutes a decision-relevant uncertainty. A taxonomy of domains in which uncertainty may exist within the regulatory-HTA interface was developed to facilitate categorization. The real-world case study was used to demonstrate how the guidance may facilitate deliberation between stakeholders and where additional guidance development may be needed.
Conclusions: The systematic approach taken for the identification of uncertainties in this guidance has the potential to facilitate understanding of uncertainty and its management across different stakeholders involved in drug development and evaluation. This can improve consistency and transparency throughout decision processes. To further support uncertainty management, linkage to suitable mitigation strategies is necessary.
Keywords: drugs; health technology assessment; regulatory decision making; stakeholder deliberation; uncertainty.
Conflict of interest statement
I.H., M.G., and M.P. are employees of MSD and stockholders of Merck & Co., Inc., Rahway, NJ, USA but act as individual contributors to this working group rather than an official company representative. I.B. is an employee of GSK and a stockholder of GSK, Philadelphia, USA but acts as an individual contributor to this working group rather than an official company representative. R.V. declares he worked part-time for the Dutch National Health Care Institute (Zorginstituut) during the conduct of this study and started working for Roche Netherlands B.V. at the finalization of the study. E.P. participated in this effort as an individual contributor rather than in the capacity afforded to her in other positions she currently holds and declares she currently serves in the Healthcare Professionals’ Working Party of the European Medicines Agency, works as a senior population health advisor for the National Organization for Healthcare Services Provision of Greece (EOPYY), and is Vice President for HTA at the European Public Health Association. C.S. is an employee and stockholder of Novartis but acts as an individual contributor to this working group rather than an official company representative. M.T. is an employee and a stockholder of IQVIA, a human data science company, but acts as an individual contributor. D.V. is a full-time employee and shareholder of Medtronic but acts as an individual contributor to this working group rather than an official company representative. W.O. and B.R. declare that DIA arranged their travel and accommodation to attend the DIA Europe 2022 conference. The other authors declare no commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- O’Rourke B, Oortwijn W, Schuller T, International Joint Task Group. The new definition of health technology assessment: A milestone in international collaboration. Int J Technol Assess Health Care. 2020;36(3):187–190. - PubMed
-
- Eichler HG, Bloechl-Daum B, Brasseur D, et al. The risks of risk aversion in drug regulation. Nat Rev Drug Discov. 2013;12(12):907-916. - PubMed
-
- Knight FH. Risk, uncertainty and profit. Boston, MA: Houghton Mifflin Co; 1921.
-
- Uncertainty [Internet]. [cited 2022 Aug 3]. Available from: https://dictionary.cambridge.org/dictionary/english/uncertainty.
-
- White GO, Boddewyn JJ, RMN Galang. Legal system contingencies as determinants of political tie intensity by wholly owned foreign subsidiaries: Insights from the Philippines. J World Bus. 2015;50(2):342-356.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

