A pH‑responsive hyaluronic acid nano‑vehicle co‑encapsulating doxorubicin and all‑trans retinoic acid for the inhibition of hepatic stellate cell‑induced tumor growth and metastasis
- PMID: 37326031
- PMCID: PMC10308492
- DOI: 10.3892/mmr.2023.13029
A pH‑responsive hyaluronic acid nano‑vehicle co‑encapsulating doxorubicin and all‑trans retinoic acid for the inhibition of hepatic stellate cell‑induced tumor growth and metastasis
Abstract
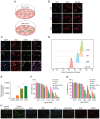
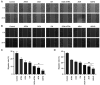
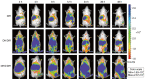
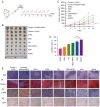
All‑trans retinoic acid (ATRA) has been implicated in the differentiation of hepatic stellate cells (HSCs). In the present study, the liver‑targeting hyaluronic acid micelles (ADHG) were prepared for co‑delivery of ATRA and doxorubicin (DOX) to block the HSC‑hepatoma interrelation. To simulate the tumor microenvironment, an in vitro dual‑cell model and an in vivo co‑implantation mouse model were established for anticancer studies. The experimental methods involved the MTT assay, wound‑healing assay, cellular uptake, flow cytometry and and in vivo antitumor study. The results revealed that the HSCs in the research models notably promoted tumor proliferation and migration. Furthermore, ADHG were readily internalized by cancer cells and HSCs simultaneously, and widely distributed in cancer regions. The in vivo antitumor studies demonstrated that ADHG could notably decrease HSC activation and extracellular matrix deposition, as well as constrain tumor growth and metastasis. Therefore, ATRA could facilitate DOX‑induced anti‑proliferation and anti‑metastasis effects, and ADHG are a promising nano‑sized formulation for the combination therapy of hepatocellular carcinoma.
Keywords: all‑trans retinoic acid; anti‑hepatoma; combination therapy; hepatic stellate cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Combined anti-hepatocellular carcinoma therapy inhibit drug-resistance and metastasis via targeting "substance P-hepatic stellate cells-hepatocellular carcinoma" axis.Biomaterials. 2021 Sep;276:121003. doi: 10.1016/j.biomaterials.2021.121003. Epub 2021 Jul 9. Biomaterials. 2021. PMID: 34273686
-
Therapeutic Response of Multifunctional Lipid and Micelle Formulation in Hepatocellular Carcinoma.ACS Appl Mater Interfaces. 2022 Oct 12;14(40):45110-45123. doi: 10.1021/acsami.2c10446. Epub 2022 Sep 27. ACS Appl Mater Interfaces. 2022. PMID: 36167351
-
Quiescent hepatic stellate cells induce toxicity and sensitivity to doxorubicin in cancer cells through a caspase-independent cell death pathway: Central role of apoptosis-inducing factor.J Cell Physiol. 2020 Sep;235(9):6167-6182. doi: 10.1002/jcp.29545. Epub 2020 Jan 24. J Cell Physiol. 2020. PMID: 31975386
-
Therapeutic modulators of hepatic stellate cells for hepatocellular carcinoma.Int J Cancer. 2020 Sep 15;147(6):1519-1527. doi: 10.1002/ijc.32899. Epub 2020 Mar 5. Int J Cancer. 2020. PMID: 32010970 Review.
-
Deciphering the possible reciprocal loop between hepatic stellate cells and cancer cells in the tumor microenvironment of the liver.Crit Rev Oncol Hematol. 2023 Feb;182:103902. doi: 10.1016/j.critrevonc.2022.103902. Epub 2023 Jan 5. Crit Rev Oncol Hematol. 2023. PMID: 36621514 Review.
Cited by
-
Exploring the Potentials of Hyaluronic Acid-coated Polymeric Nanoparticles in Enhanced Cancer Treatment by Precision Drug Delivery, Tackling Drug Resistance, and Reshaping the Tumour Micro Environment.Curr Med Chem. 2025;32(20):3960-3999. doi: 10.2174/0109298673302510240328050115. Curr Med Chem. 2025. PMID: 38571347 Review.
References
-
- Hisamori S, Tabata C, Kadokawa Y, Okoshi K, Tabata R, Mori A, Nagayama S, Watanabe G, Kubo H, Sakai Y. All-trans-retinoic acid ameliorates carbon tetrachloride-induced liver fibrosis in mice through modulating cytokine production. Liver Int. 2008;28:1217–1225. doi: 10.1111/j.1478-3231.2008.01745.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

