A cathepsin C-like protease mediates the post-translation modification of Toxoplasma gondii secretory proteins for optimal invasion and egress
- PMID: 37326431
- PMCID: PMC10470614
- DOI: 10.1128/mbio.00174-23
A cathepsin C-like protease mediates the post-translation modification of Toxoplasma gondii secretory proteins for optimal invasion and egress
Abstract
Microbial pathogens use proteases for their infections, such as digestion of proteins for nutrients and activation of their virulence factors. As an obligate intracellular parasite, Toxoplasma gondii must invade host cells to establish its intracellular propagation. To facilitate invasion, the parasites secrete invasion effectors from microneme and rhoptry, two unique organelles in apicomplexans. Previous work has shown that some micronemal invasion effectors experience a series of proteolytic cleavages within the parasite's secretion pathway for maturation, such as the aspartyl protease (TgASP3) and the cathepsin L-like protease (TgCPL), localized within the post-Golgi compartment and the endolysosomal system, respectively. Furthermore, it has been shown that the precise maturation of micronemal effectors is critical for Toxoplasma invasion and egress. Here, we show that an endosome-like compartment (ELC)-residing cathepsin C-like protease (TgCPC1) mediates the final trimming of some micronemal effectors, and its loss further results in defects in the steps of invasion, egress, and migration throughout the parasite's lytic cycle. Notably, the deletion of TgCPC1 completely blocks the activation of subtilisin-like protease 1 (TgSUB1) in the parasites, which globally impairs the surface-trimming of many key micronemal invasion and egress effectors. Additionally, we found that Toxoplasma is not efficiently inhibited by the chemical inhibitor targeting the malarial CPC ortholog, suggesting that these cathepsin C-like orthologs are structurally different within the apicomplexan phylum. Collectively, our findings identify a novel function of TgCPC1 in processing micronemal proteins within the Toxoplasma parasite's secretory pathway and expand the understanding of the roles of cathepsin C protease. IMPORTANCE Toxoplasma gondii is a microbial pathogen that is well adapted for disseminating infections. It can infect virtually all warm-blooded animals. Approximately one-third of the human population carries toxoplasmosis. During infection, the parasites sequentially secrete protein effectors from the microneme, rhoptry, and dense granule, three organelles exclusively found in apicomplexan parasites, to help establish their lytic cycle. Proteolytic cleavage of these secretory proteins is required for the parasite's optimal function. Previous work has revealed that two proteases residing within the parasite's secretory pathway cleave micronemal and rhoptry proteins, which mediate parasite invasion and egress. Here, we demonstrate that a cathepsin C-like protease (TgCPC1) is involved in processing several invasion and egress effectors. The genetic deletion of TgCPC1 prevented the complete maturation of some effectors in the parasites. Strikingly, the deletion led to a full inactivation of one surface-anchored protease, which globally impaired the trimming of some key micronemal proteins before secretion. Therefore, this finding represents a novel post-translational mechanism for the processing of virulence factors within microbial pathogens.
Keywords: Toxoplasma gondii; aminopeptidase; apicomplexan; cathepsin C; digestive vacuole; egress; invasion; lysosome; protease; protein trafficking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



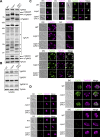

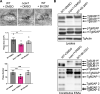
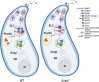
Update of
-
A cathepsin C-like protease post-translationally modifies Toxoplasma gondii secretory proteins for optimal invasion and egress.bioRxiv [Preprint]. 2023 Jan 22:2023.01.21.525043. doi: 10.1101/2023.01.21.525043. bioRxiv. 2023. Update in: mBio. 2023 Aug 31;14(4):e0017423. doi: 10.1128/mbio.00174-23. PMID: 36712013 Free PMC article. Updated. Preprint.
Similar articles
-
A cathepsin C-like protease post-translationally modifies Toxoplasma gondii secretory proteins for optimal invasion and egress.bioRxiv [Preprint]. 2023 Jan 22:2023.01.21.525043. doi: 10.1101/2023.01.21.525043. bioRxiv. 2023. Update in: mBio. 2023 Aug 31;14(4):e0017423. doi: 10.1128/mbio.00174-23. PMID: 36712013 Free PMC article. Updated. Preprint.
-
The critical role of Toxoplasma gondii GRA1 in nutrient salvage.mBio. 2025 Aug 13;16(8):e0124225. doi: 10.1128/mbio.01242-25. Epub 2025 Jun 27. mBio. 2025. PMID: 40576341 Free PMC article.
-
The Plasmodium falciparum homolog of Vps16 interacts with the core members of the Vps-C tethering complex.mSphere. 2025 Jul 29;10(7):e0028725. doi: 10.1128/msphere.00287-25. Epub 2025 Jul 8. mSphere. 2025. PMID: 40626728 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Lagal V, Binder EM, Huynh M-H, Kafsack BFC, Harris PK, Diez R, Chen D, Cole RN, Carruthers VB, Kim K. 2010. Toxoplasma gondii protease TgSUB1 is required for cell surface processing of micronemal adhesive complexes and efficient adhesion of tachyzoites. Cell Microbiol 12:1792–1808. doi:10.1111/j.1462-5822.2010.01509.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
