Antisense Oligonucleotide Activation via Enzymatic Antibiotic Resistance Mechanism
- PMID: 37326511
- PMCID: PMC10592181
- DOI: 10.1021/acschembio.3c00027
Antisense Oligonucleotide Activation via Enzymatic Antibiotic Resistance Mechanism
Abstract
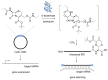
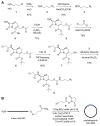
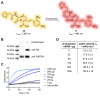
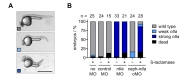
The structure and mechanism of the bacterial enzyme β-lactamase have been well-studied due to its clinical role in antibiotic resistance. β-Lactamase is known to hydrolyze the β-lactam ring of the cephalosporin scaffold, allowing a spontaneous self-immolation to occur. Previously, cephalosporin-based sensors have been developed to evaluate β-lactamase expression in both mammalian cells and zebrafish embryos. Here, we present a circular caged morpholino oligonucleotide (cMO) activated by β-lactamase-mediated cleavage of a cephalosporin motif capable of silencing the expression of T-box transcription factor Ta (tbxta), also referred to as no tail a (ntla), eliciting a distinct, observable phenotype. We explore the use of β-lactamase to elicit a biological response in aquatic embryos for the first time and expand the utility of cephalosporin as a cleavable linker beyond targeting antibiotic-resistant bacteria. The addition of β-lactamase to the current suite of enzymatic triggers presents unique opportunities for robust, orthogonal control over endogenous gene expression in a spatially resolved manner.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Deciphering the Evolution of Cephalosporin Resistance to Ceftolozane-Tazobactam in Pseudomonas aeruginosa.mBio. 2018 Dec 11;9(6):e02085-18. doi: 10.1128/mBio.02085-18. mBio. 2018. PMID: 30538183 Free PMC article.
-
Exploitation of Antibiotic Resistance as a Novel Drug Target: Development of a β-Lactamase-Activated Antibacterial Prodrug.J Med Chem. 2019 May 9;62(9):4411-4425. doi: 10.1021/acs.jmedchem.8b01923. Epub 2019 May 1. J Med Chem. 2019. PMID: 31009558 Free PMC article.
-
Beta-lactamases and beta-lactamase inhibitors.Int J Antimicrob Agents. 1999 Aug;12 Suppl 1:S3-7; discussion S26-7. doi: 10.1016/s0924-8579(99)00085-0. Int J Antimicrob Agents. 1999. PMID: 10526867 Review.
-
Insignificant β-lactamase activity of human serum albumin: no panic to nonmicrobial-based drug resistance.Lett Appl Microbiol. 2013 Oct;57(4):325-9. doi: 10.1111/lam.12116. Epub 2013 Jul 3. Lett Appl Microbiol. 2013. PMID: 23758063
-
Penicillin-Binding Proteins, β-Lactamases, and β-Lactamase Inhibitors in β-Lactam-Producing Actinobacteria: Self-Resistance Mechanisms.Int J Mol Sci. 2022 May 18;23(10):5662. doi: 10.3390/ijms23105662. Int J Mol Sci. 2022. PMID: 35628478 Free PMC article. Review.
Cited by
-
Chemical strategies for antisense antibiotics.Chem Soc Rev. 2024 Nov 25;53(23):11303-11320. doi: 10.1039/d4cs00238e. Chem Soc Rev. 2024. PMID: 39436264 Free PMC article. Review.
-
Frosted DNA: β-Galactosidase Control of Oligonucleotide Activity.Chemistry. 2025 Apr;31(24):e202500347. doi: 10.1002/chem.202500347. Epub 2025 Mar 30. Chemistry. 2025. PMID: 40084398 Free PMC article.
-
Direct Activation of Nucleobases with Small Molecules for the Conditional Control of Antisense Function.Angew Chem Int Ed Engl. 2024 Apr 22;63(17):e202318773. doi: 10.1002/anie.202318773. Epub 2024 Mar 15. Angew Chem Int Ed Engl. 2024. PMID: 38411401 Free PMC article.
-
Restoring susceptibility to aminoglycosides: identifying small molecule inhibitors of enzymatic inactivation.RSC Med Chem. 2023 Jul 21;14(9):1591-1602. doi: 10.1039/d3md00226h. eCollection 2023 Sep 19. RSC Med Chem. 2023. PMID: 37731693 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

