Independent Tuning of the p Ka or the E1/2 in a Family of Ruthenium Pyridine-Imidazole Complexes
- PMID: 37326619
- PMCID: PMC10734561
- DOI: 10.1021/acs.inorgchem.3c01241
Independent Tuning of the p Ka or the E1/2 in a Family of Ruthenium Pyridine-Imidazole Complexes
Abstract
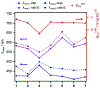
Two series of RuII(acac)2(py-imH) complexes have been prepared, one with changes to the acac ligands and the other with substitutions to the imidazole. The proton-coupled electron transfer (PCET) thermochemistry of the complexes has been studied in acetonitrile, revealing that the acac substitutions almost exclusively affect the redox potentials of the complex (|ΔE1/2| ≫ |ΔpKa|·0.059 V) while the changes to the imidazole primarily affect its acidity (|ΔpKa|·0.059 V ≫ |ΔE1/2|). This decoupling is supported by DFT calculations, which show that the acac substitutions primarily affect the Ru-centered t2g orbitals, while changes to the py-imH ligand primarily affect the ligand-centered π orbitals. More broadly, the decoupling stems from the physical separation of the electron and proton within the complex and highlights a clear design strategy to separately tune the redox and acid/base properties of H atom donor/acceptor molecules.
Figures


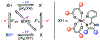
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

